Types of BatteryNowadays, most electronic devices are using batteries. Batteries help make most electrical and electronic devices portable to be used where there is no electricity. Most people know that there are two types of batteries, chargeable and non-chargeable. But in fact, there are many different types of batteries under the chargeable and non-chargeable category. We can also say that batteries are the backbone of wireless electronic devices and equipment. In this article, we are discussing different types of batteries and their characteristics. Before discussing the battery types, let's first understand the definition of battery: What is a Battery?Each battery is typically a type of galvanic cell in which multiple redox reactions are carried out between two electrodes. These electrodes act as sources of chemical energy. A battery is a chemical device used to store electrical energy as chemicals and through an electrochemical reaction. Electrochemical reaction helps to transfer electrons from one substance (electrode plate) to another through electrical circuits. This means that the stored chemical energy is converted into a direct electrical current or electrical energy within the cell. A battery may have one or more cells arranged in a series/parallel mode depending on the voltage level requirements. The most common example of a battery is a power bank that helps to charge other portable gadgets such as smartphones, wireless earphones, etc. Note: It is important to note that the battery can only store DC (direct current). They are not efficient to be used to store AC (alternating current). This is why direct current is mainly used for portable electronic devices.What is a cell?Although batteries are known for storing chemical energy, the basic unit responsible for the actual energy storage is the battery's cell. A cell refers to the fundamental electrochemical unit that acts as a substantial electrical energy source generated by chemical energy conservation. The cell is constructed using three main components, such as an electrolyte and two electrodes. However, it also includes terminals, containers, separators, and other components. Speaking of electrodes, a cell consists of two different electrodes, such as anode (negative) and the cathode (positive). The anode transfers electrons to the external circuit, which are further oxidized during the electrochemical reaction. The cathode accepts electrons from the internal circuit, which are reduced during the electrochemical reaction. Therefore, the conservation of energy in a cell is due to the electrochemical oxidation-reduction reaction. Apart from this, the third component, the electrolyte, plays the transfer medium's role for charges (ions) between the anode and the cathode. Types of BatteryAlthough there are many types of batteries, they are broadly classified into the following two types:
However, they can be classified differently based on their applications. Let us now understand both the above significant types: Primary BatteriesPrimary batteries are those that do not support the adoption of recharge mechanisms. Once these batteries are discharged, they cannot be charged again. These types of batteries have electrochemical cells, in which the electrochemical reaction cannot be reversed. In particular, redox reactions in primary batteries proceed in only one direction. The reactants present in these batteries are consumed after a specific period, leading to their death. Therefore, once the chemicals are exhausted, the primary battery is dead and cannot be used to operate supported devices. Primary batteries are designed primarily for devices that can operate in relatively small amounts of power supplies. This ultimately makes these batteries durable or long-lasting to the maximum extent possible. Primary batteries are often referred to as non-rechargeable batteries or disposable batteries. 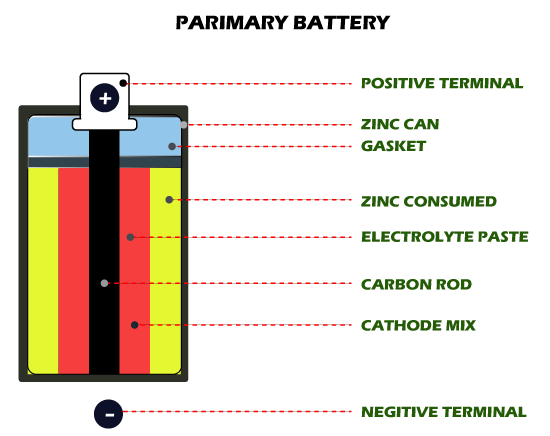
The following are the types of commonly used primary batteries: Alkaline BatteriesAlkaline batteries are a type of batteries manufactured primarily using the chemical composition of zinc and manganese dioxide. In these batteries, potassium hydroxide participates as an electrolyte. Potassium hydroxide used in these batteries is a purely alkaline material, and therefore the manufactured battery is known as an alkaline battery. Alkaline batteries are widely used in remote controllers, torch lights, wall clocks, and many other portable toys and devices. Typically, the power density of these batteries is about 100 Wh / Kg. 
In terms of advantages, alkaline batteries are known for their small size, efficient performance, maximum cycle life, good shelf life, and low risk of leakage. They typically have low internal resistance, which helps these batteries retain their energy stored in the inactive state. Besides, their cost may seem very high according to their lifespan. Coin Cell BatteriesCoin cell batteries are batteries that contain cells whose chemical composition is also alkaline. However, lithium and silver oxide chemicals are also used to make these batteries. These chemicals help to provide stable or steady voltage to these batteries even with minimal size. Coil cell batteries are widely used in wristwatches, calculators, table clocks, and small electronic devices. Typically, the power density of these batteries is about 270 Wh / Kg. 
In terms of advantages, coin cell batteries are lightweight, small, inexpensive, and have a durable shelf life. Besides, they can achieve high nominal voltages up to 3 V when cells are arranged chronologically. However, these types of batteries require a holder and usually have a low current draw capability. Apart from the above two types of primary batteries, some of the rarely used primary types of batteries are tabulated below, including their features and applications:
Secondary BatteriesSecondary batteries are those that are supported to adopt recharge mechanisms. These batteries contain electrochemical cells in which chemical reactions can be reversed by applying a particular voltage to the battery in the reverse direction. In simple terms, secondary batteries tend to recharge after passing a current through the electrode in the opposite direction within the circuit, meaning from the negative terminal to the positive terminal. Secondary batteries are typically used in two different ways. In the first category of application, these batteries are charged by connecting them to a power source and simultaneously supplying energy to other devices. Besides, the second category includes secondary batteries that can be either charged or used at a time. This means they are first used as primary batteries until they are discharged and then recharged back to their original state using some charging mechanism. Secondary batteries are also referred to as chargeable or rechargeable batteries. 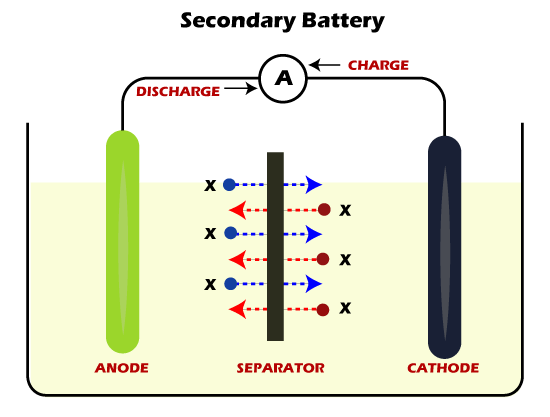
Although secondary batteries have higher power density, they have lower energy density than the primary batteries. Also, the secondary batteries have low-temperature performance and a high discharge rate. The following are the types of commonly used secondary batteries: Lead-acid BatteriesAs the name suggests, lead-acid batteries are manufactured using lead-acid as the main components, which is relatively inexpensive. Most of these batteries are available in various sizes and are mainly used to power lighting in vehicles. Other applications of these batteries include robotics, machinery, etc. 
Lead-acid batteries usually come with a nominal voltage, starting at 2V. For example, 2V, 6V, 12V, and 24V. Typically, the power density of these batteries is about 7 Wh / Kg. Although lead-acid batteries have low power density, they have high power output capacity. Ni-Cd (Nickel Cadmium) BatteriesNi-Cd batteries are manufactured as the base chemical composition of nickel and cadmium. These batteries usually come in all standard sizes, such as AA, AAA, C, and rectangular. They are low in price and can work in all environments. Besides, their discharge rate is relatively low compared to nickel-metal hydride batteries. These batteries are mainly used in solar lights, cordless phones, and RC toys. 
Ni-Cd batteries usually come with a nominal voltage of 1.2V. However, they are often used in a set of 3 batteries that provide 3.6 V combined. Typically, the power density of these batteries is about 60 Wh / Kg. Ni-MH (Nickel-Metal Hydride) BatteriesNi-MH batteries are manufactured using nickel-metal hydride and are preferred over Ni-Cd batteries as they do not affect the environment as much as Ni-Cd batteries. Like Ni-CD batteries, Ni-MH batteries are also available in standard sizes. Ni-MH batteries are an excellent alternative to Ni-CD batteries due to their availability and similar features. However, they are expensive and have high self-discharge rates. These types of batteries have the same usage as alkaline and Ni-Cd batteries. 
Ni-MH batteries usually come with a nominal voltage of 1.25 V. Typically, these batteries' power density is about 100 Wh / Kg. Li-ion (Lithium-ion) BatteriesLi-ion batteries, also called lithium-ion batteries, are manufactured using lithium metal and efficient rechargeable mechanisms. These batteries are widely used in portable devices and gadgets, especially in smartphones. 
Li-ion batteries come with nominal voltages of 3.7V and 7.2V. They have a wide range of electrical capacities ranging from 100 mAh to 1000 mAh. Although Li-ion batteries are small and lightweight, they have a high charging rate (about 1 C to 10 C). Also, the power density of these batteries is very high compared to their size. They have a power density of about 100 Wh / Kg. Li-Po (Lithium-ion Polymer) BatteriesLi-Po batteries, or lithium-ion polymer batteries, are rechargeable and fall under Li-ion technology. Instead of liquid electrolyte, Li-Po batteries are manufactured using high conductivity polymer electrolyte. These are thinner, lighter, and highly protective than Li-ion batteries. However, they are costly. 
Li-Po batteries are commonly used in portable devices such as RC toys, robotics, drones, etc. Typically, the power density of these batteries is about 185 Wh / Kg. SMF (Sealed Maintenance Free) BatteriesSMF (sealed maintenance-free batteries) are manufactured primarily to provide consistent, reliable, and low maintenance power for UPS-based applications. Batteries of this type require minimal maintenance, even in power deficit locations. Moreover, they are considered to have deep cycle applications. SMF batteries are available in various voltages starting at 12V. These batteries are essential in the field of data and storage. They continue to provide a continuous power supply even after the power goes out, preventing the unexpected shutdown of devices and reducing the risk of data loss. Subsequently, SMF batteries are known to be used as a reliable and proven battery system. 
Classification of Battery based on the ApplicationIn the context of various battery applications, they are generally classified into the following three types: Household BatteriesThese are the batteries we use the most in our regular household applications. Some household batteries are rechargeable, which we can usually plug into the charger and charge for repeated use. After repeated use, they become dead and must be replaced. Besides, there are non-rechargeable household batteries that we can only use for a particular time. They are single-use type batteries, and once they are at full discharge, they need to be recycled. Such batteries do not support charging. Household batteries are usually sub-divided into the following categories:
Household batteries come in different shapes and sizes. They are used in many home appliances such as clocks, watches, cameras, torch-lights, etc. Industrial BatteriesEveryday users or individuals do not commonly use these batteries. Organizations, firms, and industries mainly use them. Batteries manufactured in this category are efficient to meet heavy-duty requirements. They find uses in a diversity of heavy applications, primarily in backup power for railroads, data centers, machinery, telecommunications, utilities, and more. Industrial batteries are mostly rechargeable and large. Some standard industrial batteries are nickel-iron batteries and wet nickel-cadmium (NiCd) batteries. Vehicle BatteriesThese batteries are known to be more user friendly and the least sophisticated version of industrial batteries. Most of these batteries are relatively small and support charging systems for a particular number of cycles. Vehicle batteries are widely used to power cars, bikes, boats, and many other motorized vehicles. A typical example of a vehicle battery is a lead-acid battery. Other examples include electric automotive batteries, hybrid automotive batteries, and valve-regulated lead-acid or VRLA batteries.
Next TopicTypes of Capacitor
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









