Conductor
An electric conductor is a substance that permits the flow of electric current through it.
The material that possesses the property of flow of electric current is generally metals.
The structure of a conductor consists of free electrons. It is shown below:
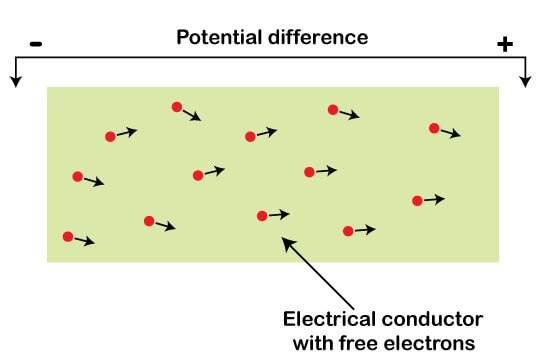
In the case of metals, the charge inside a conductor is generally free electrons. But, if we consider a battery case, it consists of both positive and negative charges that constituted the current in a battery.
If the free electrons present in a conductor can easily move, it means that it possesses the property to permit electric current flow.
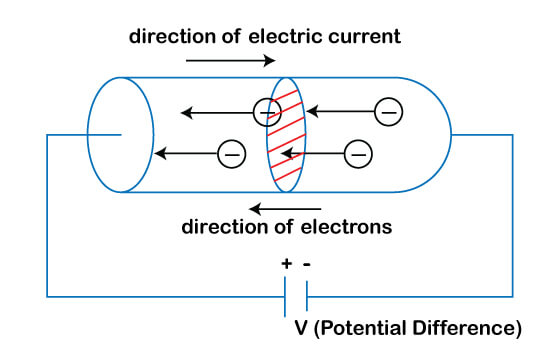
Whenever there are movement free electrons in a conductor, the electric current is created inside a conductor.
It is shown below:
Resistance of a Conductor
The conductor's resistance is inversely and directly proportional to the cross-sectional area and length of the conductor. For example, long copper wire has higher resistance than copper wire of a shorter length.
The resistance is given by:
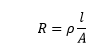
Where,
l is the length
A is the cross-sectional area of the conductor
The unit of length is meter, while the unit of area is meter square.
Conductance of a conductor
The conductance of a conductor is directly and inversely proportional to the cross-sectional area and length of the conductor.
The conductance is given by:
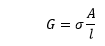
Where,
l is the length
A is the cross-sectional area of the conductor
Conductance is also defined as the reciprocal of resistance.
G = 1/R
But, we are often confused between conductance and conductivity. Let' discuss the difference between them.
The conductance of a conductor is the extrinsic property that is based on the object, while conductivity is the intrinsic property based on the material.
Conductive coating
A coating is applied on the surface of the object. The purpose may be decorative or functional.
The coating can be a partial coating on the surface, full coating, or all-over coating. Conducting coating over a surface is used as circuitry. It is also applied on the insulators or non-conducting material to permit the current flow through their surface. Metal conductive coatings are also applied to the metals to protect them from water and air. Otherwise, the metal may corrode and rust when exposed to environmental substances.
Let's discuss the coating materials used in the process of the conductive coating.
Tin
Tin is a popular coating material due to its low cost and low melting point. But, the tin is less conductive as compared to cooper.
Silver
It is highly recommended to reduce attenuation and impedance. It is also used at high temperatures and in high-frequency applications. It can form an oxide layer even at normal temperatures.
Nickel
Nickel is used as conductive plating. The nickel coating adds luster and brightness to the object. It also protects the substance from corrosion and abrasion. It is used in various applications, such as engineering and decorative finishing.
Aluminum
The coatings of aluminum have better protection from corrosion as compared to zinc coating. It is generally used as a base material to protect the substance from external environmental factors.
Copper
Copper is considered as a good conductor of electricity. So, cooper coatings are preferred to increase the conductivity of a substance.
Functions of conductive coatings
The functions of conductive coatings are as follows:
- Reflective coating for the use in mirrors
- Prevention from corrosion and rust
- Wear resistance
- Coating maintenance for alloys
- Anti-friction coating
- Increased conductivity
- Prevention from degradation
- Manufacturing of resistors
Examples of conductor
Here, we will discuss the examples of the conductor. We will also discuss the reason behind its conductive properties.
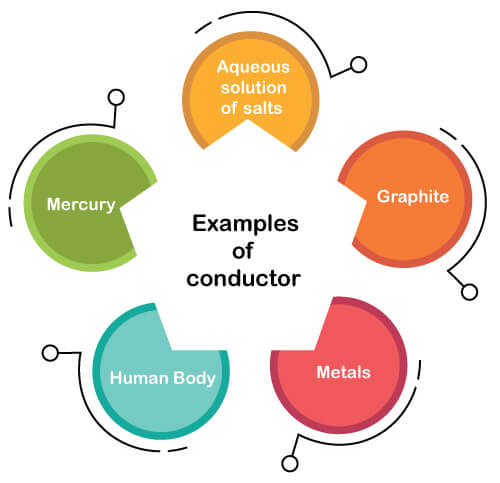
The examples of a conductor are given below:
- An aqueous solution of salts
The aqueous solution of salt comprises of Sodium (Na+) and Chloride (Cl-) ions. Salt or NaCl is a combination of metal and non-metal, which together form an ionic bond. The ions of salt have an electrical charge. The aqueous solution of salt (salt in water) allows ions to move freely in the solution. The aqueous solution formed can conduct electricity due to the presence of charged sodium and chloride ions. Hence, an aqueous solution of salt behaves as a conductor.
- Graphite
The structure of graphite consists of delocalized electrons similar to that of metals. These electrons can easily move between the layers of graphite. The forces between its layers are weak, which allows the structure to conduct electricity. Hence, graphite works as a conductor.
- Metals
Copper is also known as a good conductor. It has the highest electrical conductivity as compared to other non-precious metals. The conducting electrons in a metal can easily move, which allows the flow of current through the metal. Hence, copper metal works as a conductor.
Silver has the highest conductivity compares to all other metals.
Similarly, other metals such as aluminum, silver, gold, steel, brass, etc., are called conductors.
- Human Body
We know that ions conduct electricity. The human body contains different ions, such as potassium ions, iron, sodium ions, etc. The presence of ions in the human body allows the flow of current through it. Hence, the human body can conduct electricity.
- Mercury
Mercury is a metal in liquid form. The color of mercury is generally silver-white. It is considered a poor conductor of heat. It is called a fair conductor. Mercury can easily alloy with other types of metals, such as silver and tin. Such metals' melting points are low, which allows them to stay in liquid form at room temperature.
Applications of Conductor
It means the use of conducting materials in our life. We all use conductors in everyday life, which are called good conductors. Let's discuss the different applications of conductors.
- Utensils
Metals are good conductors of electricity as well as heat. Utensils are made of metals, such as aluminum, copper, and steel. It easily allows heat to pass from the base to the substance inside the utensil. The handles of made of bad conductors for easy holding.
- Clothing Iron
The base of the iron is made of metal that generates heat when connected to an electric supply. The metal present at the bottom of the iron is in direct contact with the cloth. The top of the iron is an insulator for easy holding and current protection.
- Current Tester
The tester is the material used to test the flowing current in the wires. The outer material of the tester is made of insulators. The tip of the tester is made in touch with the conductor. If it detects the current, a small neon lamp will light inside a tester.
- Cooking Oven
Baking tools and cooking oven are made from conductive materials. It uses electricity to generate heat, which is used to ripe the food kept inside it. The oven equally distributes heat in all directions to cook the food.
- Soldering
Soldering is a process to attach and repair electronic equipment. The bottom of the soldering machine is a conductor that comes in contact with the tool to be soldered.
- Conducting wires
The conducting wires help in the transmission of electricity over long distances. The commonly used metals are aluminum, copper, etc.
Copper is the most used metal in creating conducting wires due to its flexibility and low resistance.
- Electric kettles
The base sheet of kettles is made of steel. The steel is generally stainless. The kettle can also be composed of other metals, such as copper. Stainless steel is preferred over other metals because it has high durability, can withstand scrubbing, does not discolor, and causes no threat.
- Pistons
Pistons are a part of various devices, such as compressors, cylinders, engines, etc. The pistons are used to convert the pressure into motion. Pistons are created from conducting materials like aluminum alloys or carbon steel.
Carbon steel material is preferred because it reduces the thermal expansion between cylindrical walls and pistons.
Non-conductive Materials or Insulators
The materials that do not allow the flow of current through it are called non-conducting materials or insulators. Such materials block the flow of current when placed in a circuit. The insulating material is used to support electrical appliances to prevent people from electrical shock and leakage. Insulators have high resistance as compared to semiconductors and conductors. The insulating materials are generally called non-metals.

Let' discuss some differences between conductor and Insulator. It is shown in the below table:
| Category |
Conductors |
Insulators |
| Definition |
It easily allows the movement of electrons in it. |
The movement of electrons in insulators is difficult. |
| Resistance |
Low |
High |
| Magnetic energy |
Conductors can store magnetic energy. |
Insulators cannot store magnetic energy. |
| Thermal Conductivity |
High |
Low |
| Forbidden gap |
There is no forbidden gap. The valence band and conduction band of conductors overlap each other. |
The forbidden gap between the valence band and conduction band is large in insulators. |
| Applications |
Electrical wires, etc. |
Insulators are used in electrical equipment to save people from high current. |
| Examples |
Aluminum, copper, etc. |
Wood, paper, plastic, etc. |
|


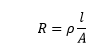
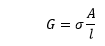


 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now









