Difference between atoms and moleculesThere is an interconnection between atoms and molecules in a certain way. Atom is the tiniest unit that constitutes a chemical element. Molecules are collections of two or more chemically bonds atoms. Two general terms are atom and molecule. In this subject, we will explore the differences between atoms and molecules first and then discuss them. 
Atoms (Definition and Signification)The atom is the smallest particle of an element or compound. Besides, it can or cannot exist independently. It consists of the nucleus and the subatomic particles (protons and neutrons). It has a circular form. The nucleus of the atom is located at center and is encircled by negatively charged electrons. Also, the proton and neutrons are in the nucleus together and are almost identical in weight. E.g., N, H, He, O, K, etc. 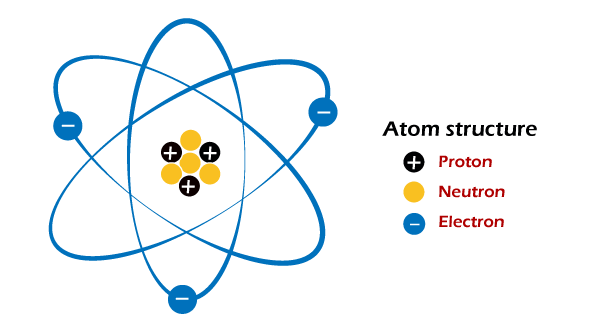
MoleculesMolecule is a collection of two or more bonding atoms. They also exist in a free state and consist of two or more similar or non-identical bound atoms. Molecular electrons can react with each other, resulting in a chemical reaction with this affinity. The most noticeable thing is that the electrons are swapped between elements in the molecular connection. E.g., CO2, NaCl, HCl, NO2, and O3 Everything we see and feel is the matter. This matter, which consists of people, animals, plants, and other non-living substances like water and rocks, is constituted by very trivial particles considered to be the basic building block of a substance. You must have heard your instructor speaking about atoms and molecules, if you were attentive throughout your science lessons in school. But the problem is most likely that few of you can recall these things. Throughout chemistry, there is indeed a significant question: how the atoms and molecules differ from each other?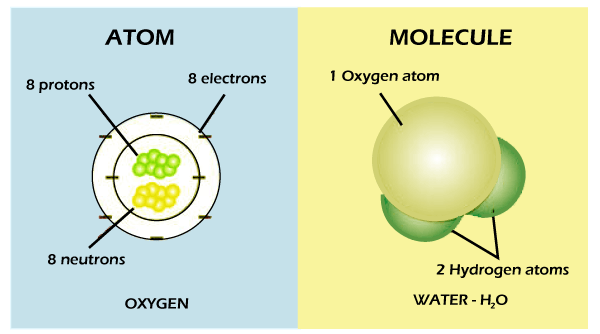
The distinction between the atom and the molecule is whether an atom is trivial in any component and has the characteristics. It is impossible to break down an atom and keep the element's characteristics. Therefore, the collective power of two or more atoms is at the expense of molecules. Thus, the collective forces of two or more atoms have been at the expense of molecules on either side. One of the main distinctions between molecules and atoms is that the former could be split further. Water would have been an instance of atoms and molecules. It is a molecule (H2O) that contains 2 hydrogen atoms and one oxygen atom. When you speak about an atom, it refers to the smallest particle that is a chemical in any ordinary stuff. Anything soluble in the atmosphere around you is made up of charged or neutral atoms. The atom's usual size is around 100 picometer, about 1/millionth in a millimeter. Because the atoms are pretty tiny, predicting their behavior using conventional physics is not an easy process. So instead, experts use quantum concepts to explain the behavior of atoms. In each atom, you'll discover a nucleus with at least one electron that is connected to the nucleus. This nucleus, in turn, consists of at least one proton and many neutrons. The protons and neutrons are considered nucleons, representing approximately 99.94 percent of the entire mass of nucleus. The protons produce positive charges, and the electrons generate negative charges. The neutrons have no charge at all. If at least two atoms are linked with the help of chemical bonds, it produces an asymmetric molecule. Thus, you can most often declare that molecules are like each other's ions. Well, between these two: Ions are energized, whereas electrostatic interactions substances are not. You will observe that not only organic chemistry, biochemistry, and quantum physics are not applied. You must be aware of the nuclear nature of molecules. In other words, one atom may be included from a single organic compound in a molecule.
While the atoms and the molecules are tiny and can't be seen with the human eye, both do not indicate that the characteristics are identical. Atoms are less and the essential components of molecules. Because of electrons, atoms are energized electronically, but the molecules are not energized electrically. Even the atomic and molecular forms are distinct.
Next TopicDifference between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










