Difference between Cell and BatteryHistory of Cell and BatteryIn recent years, there has been a surge in interest in generating alternative energy sources and figuring out how to store energy from renewable sources like wind and solar. Batteries have been critical, with new technologies for storing and delivering energy from renewable sources. There have been constant inventions and progress in the field of cells and batteries till now, From Alessandro Volta's early 19th-century invention of the first genuine battery to the current Li-ion batteries utilized in today's technological devices, the growth of batteries has been driven by the demand for portable, efficient, and reliable sources of energy. Many of you might have heard about cells and batteries, but what is the difference between them? Are these the same or different? Let us discuss various essential details related to them. Difference between Cell and BatteryThe battery and cell are essential inventions that have simplified our daily lives and duties. They are found in almost all portable electronic gadgets that we use nowadays. We might also remark that we cannot imagine a world without batteries and cells. However, did you know that a cell and a battery are not the same things, despite the fact that the terms are commonly used interchangeably? A battery often includes pre-supplied electrical energy from the factory, or the battery may be charged via an outlet. On the other hand, a cell takes chemical energy sources such as natural gas, diesel, or propane and processes them into electrical energy for electricity. 
What exactly is a Cell?A cell is a single power-generating device that stores chemical energy and subsequently transforms it into electrical energy. A cell is an electrochemical device that generates energy through chemical processes. The biggest benefit of cells is that they provide a portable source of electrical energy. An electrolyte (a chemical substance) in the cell interacts with the electrodes to create an electric current. A cell contains two electrodes known as the cathode and anode. The redox reaction occurs between the electrolytes and the electrodes, causing electric current to flow via the external circuit. The anode is the site of the oxidation process, whereas the cathode is the site of the reduction reaction. The energy in a cell is stored until it is utilized. It is an efficient method of storing electrical energy since it does not lose energy over time while not in use, which is why it is extensively employed in domestic appliances. Because it is a unit, a cell is often light in weight and small in size. It is inexpensive; however, it only provides energy or power for a limited time. Electrons are received from an external generator and stored in a cell for eventual use. Cells are used in low-energy devices such as clocks, radios, lighting, remote controls, and so on. Below is the flowchart of differentiation of cell and battery:- 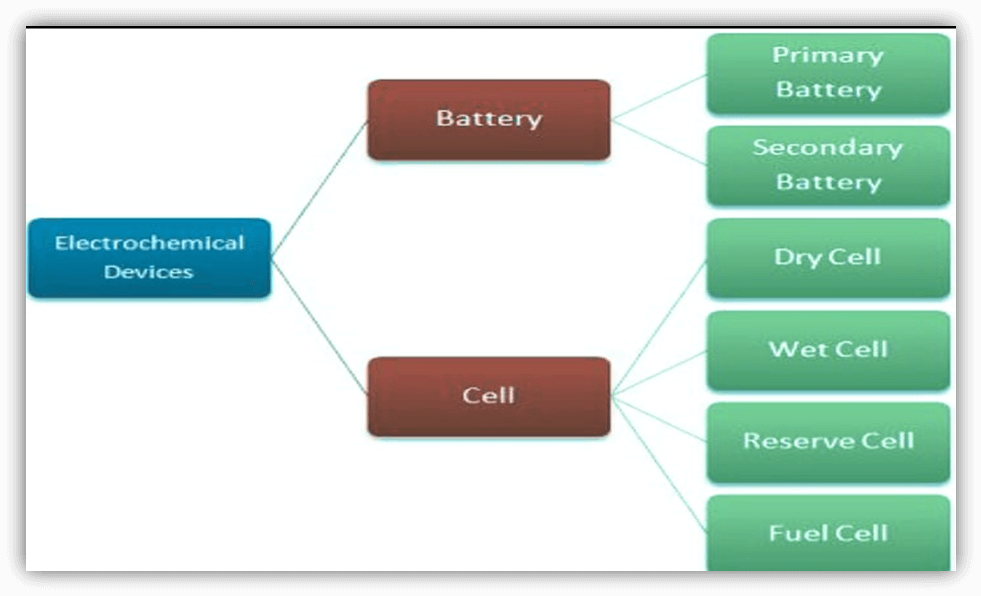
Some of the most popular types of electric cells are as follows:Primary Cell: The energy in these cells is converted from chemical to electrical. These are undergoing an irreversible chemical process. These cells are exclusively capable of discharge. Their internal resistance ranges between one and two ohms. Those cells are incapable of carrying a high current. Simple voltaic cells, Leclanche cells, Daniel cells, dry cells, and so on are examples. Secondary Cell: In these cells, electrical energy is turned into chemical energy, which is then transformed back into electrical energy, additionally known as storage cells. These cells may be charged or non-charged. They are undergoing a reversible chemical reaction. Also, the cell's intrinsic resistance ranges from 0.1 to 0.001 Ohm; cells may conduct a high current. Lead acid accumulators and nickel metal hydrides are some examples. Dry Cells: These batteries are sealed and have an electrolyte that resembles a paste rather than a liquid. Because they are not prone to leakage or spilling, they are perfect for use in portable devices. Alkaline batteries, nickel-metal hydride batteries, and zinc-carbon batteries are some examples of dry cells. Wet Cells: Wet cells are liquid-electrolyte batteries that are regularly utilized in large-scale applications such as backup power systems and electric automobiles. Lead-acid batteries, nickel-cadmium batteries, and sodium-sulfur batteries are examples of wet cells. Fuel Cell: The fuel cell may be described as an electrochemical device that continually transforms the chemical energy of the fuel into electricity and heat, all without the use of fire. A fuel cell is similar to an electrical cell or battery in that both include positive and negative electrodes, as well as the electrolyte in between, and they both provide direct current (DC) power. Reserve Cell: Reserve cells efficiently eliminate self-discharge and reduce chemical degradation. Typically, reserve cells are only utilized once before being removed. Reserve cells are utilized in missiles, torpedoes, and other weapon systems as time, temperature, and pressure-sensitive detonation mechanisms. What is a Battery?A battery is composed of one or more cells that are linked in series or parallel. An electrochemical device known as a battery turns chemical energy that has been stored into electrical energy that may be used to power electrical equipment and gadgets like mobile phones, flashlights, and electric vehicles. A battery is a constant power source that generates electricity in the form of a Direct Current (DC). To allow for the movement of ions between the electrodes, the electrodes must be separated and submerged in an electrolyte. The electrodes and electrolytes are chosen and configured to provide enough electromotive force and current between the battery terminals to power machines, lights, and other devices. Batteries are divided into two types: Primary and SecondaryPrimary Batteries: These are the batteries that can only be used once and cannot be reused; thus, they are often referred to as single-use batteries or throwaway batteries. Because the materials inside the batteries are reduced permanently upon discharge, they can only be used once. Secondary Batteries: Sometimes known as rechargeable batteries, may be recharged multiple times after being drained. The process of discharging and recharging is carried out using electric current, with reverse current assisting in returning the electrons to their former condition. Here is a comparison table, which can help you to clear the difference between cells and batteries.
Conclusion:By looking at both aspects of cells and batteries, we can conclude that electric cells exist in a variety of forms and sizes, each with its own set of benefits and drawbacks. When selecting a battery for a certain usage, consider aspects such as energy density, power output, safety, cost, and environmental effect. Technology advancements have accelerated the area of battery research, and new battery types are being created that provide even higher performance and efficiency. However, we can say that both are essential with different benefits.
Next TopicDifference between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









