Difference Between Evaporation and VaporizationVaporization and evaporation are two similar processes, but they have distinct properties that make them effective in different ways. The primary distinction between these two processes is that vaporizing requires an external energy source to provide sufficient heat for the transition from liquid to gas, whereas evaporation does not require any outside input if there is a temperature difference between the system and its surroundings. It should also be noted that both methods generate one type of substance into another, only vaporization converts a liquid into a gas, whereas evaporating simply changes a liquid into its vapor phase. What is Evaporation?Evaporation is the process by which a liquid turns into a gas. Have you ever noticed how quickly water evaporates from a glass left on a table? It is not hungry fairies in your kitchen, but evaporation. Evaporation is the spontaneous conversion of molecules from the liquid to the gas phase. Condensation is the inverse of evaporation. Evaporation is a kind of vaporization that occurs on liquid surfaces and involves the transfer of molecules of a liquid into the gaseous phase. As a result, this process is thought to involve a change in the liquid state of matter. 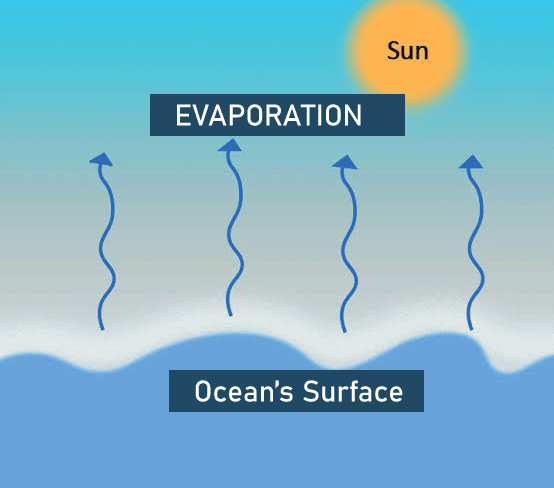
During evaporation, the molecules with the highest kinetic energy only scatter from the topmost layer of the liquid. Another property of evaporation produces a cooling effect. For example, In the summer the earthen pot was intended to store cool water because water oozed out of the minute pores of the pot, causing evaporation as well as cooling the water inside the pot. The desert cooler also works on the evaporation principle. When molecules in a liquid near its surface have enough kinetic energy to escape the interaction and move away from the liquid's body, evaporation occurs. When molecules escape, their remaining average kinetic energy decreases. This causes the liquid's temperature to drop and is the basis for the evaporative cooling phenomenon. Temperature, liquid surface area, wind speed, and humidity are all factors that impact evaporation. Process of EvaporationWhen the liquid is heated, it evaporates. This implies that the liquid's molecules must acquire kinetic energy. As kinetic energy is gained the molecules of a liquid expand and vibrate more rapidly. Therefore, the liquid changes its state to gas. When energy or heat is applied to water, the bonds that secure the molecules together begin to weaken, causing it to transit from a liquid to a gas. Water's boiling point, 212 degrees Fahrenheit or 100 degrees Celsius, is indeed the temperature at which it transitions from a liquid to a gas takes place. 
Evaporation CausesEvaporation occurs when liquid water turns into water vapor, with rivers, lakes, and oceans accounting for roughly 90% of the water undergoing this transformation. Consider a pot of boiling water to understand the cause of Evaporation better. When the water in the pot reaches 100 degrees Celsius, water vapor in the form of steam can be seen rising (212 degrees Fahrenheit). Heat is needed to separate water molecules from one another, which causes evaporation. Factors affecting EvaporationEvaporation makes our lives easier in many aspects, and it also contributes to the water cycle. However, the rate of evaporation is controlled by several factors. Another thing to remember is that Evaporation is a slow process that can be accelerated or slowed by external factors. Let us go over all these evaporation factors:
The first factor is temperature because we know that evaporation can occur at any temperature before boiling point since temperature also plays a role in evaporation. The rate of evaporation is determined by the temperature the higher the temperature, the faster the rate of evaporation. Now the question is, how? It's a known fact that increasing the temperature raises the kinetic energy, which is the energy required to break the intermolecular forces that hold the liquid molecule together. As a result, as the temperature rise, the molecule rapidly loses its intermolecular forces and evaporates. This implies that Temperature ∝ Evaporation For example, we have all noticed that on hot summer days, clothes dry faster than on normal days. This is because of the temperature.
We previously explained how Evaporation is a physical phenomenon and how the rate of Evaporation is influenced by the surface. The evaporation rate increases with increasing surface area. So, if there is more surface area then more liquid molecules will be on the surface which means more molecules will break their intermolecular bonds by increasing the evaporation rate. As a result, we can write it as. The surface area of liquid ∝ Evaporation For example, a plate with water evaporates the same amount of water faster than a shallow cup since the plate has a larger area of surface for the water than a shallow cup.
Humidity refers to the amount of moisture or vapor in the air. The higher the humidity, the more water vapor is in the air. The rate of evaporation slows as humidity rises. Humidity ∝ 1/Evaporation For example, drying our clothes becomes extremely difficult during the rainy season when our surroundings are more humid.
Evaporation is proportional to wind speed, so as the wind speed increases, therefore the rate of evaporation will also be increased. Wind Speed ∝ Evaporation For example, clothes dry faster on a windy day than on a normal day. This is because the wind reduces humidity, which increased the rate of Evaporation. VaporizationVaporization is a phase transition of an element or compound from its solid or liquid phase to the corresponding gas phase. It can also refer to the physical destruction of a substance caused by intense heat. Vaporization is the process of converting a substance from a solid or liquid state to a gas state using heat. It should be noted that these changes in matter state from one to another occur without a change in chemical composition. No chemical changes are occurring. Changes in a substance's temperature or pressure cause the changes. For example, as the temperature rises, the intramolecular interaction between the particles increases, allowing the substance to move more freely. The substances settle into a fixed and rigid structure when the temperature is reduced. Melting, freezing, deposition, sublimation, condensation, and the processes confronted in the three-state matter cycle include vaporization. 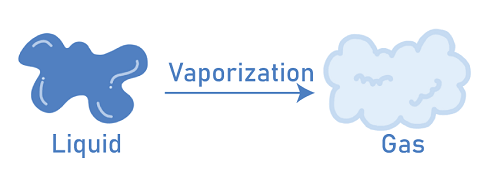
The term vaporization has also been used commonly and hyperbolically to refer to the physical destruction of a substance by intense heat or explosive force, in which the object is blown into small pieces rather than transformed into a gaseous state. In the 1952 Ivy Mike thermonuclear test, the "vaporization" of the unoccupied Marshall Island of Elugelab is an example of this usage. Many more examples can be found in MythBusters episodes involving explosives, the most notable of which include Cement Mix-Up, in which they "vaporized" a cement truck with ANFO. Latent Heat of VaporisationThe latent heat of vaporization is the amount of heat needed to simulate the conversion of one kilogram of liquid into a gas with a boiling point at a given temperature. The latent heat of vaporization varies with the liquid. As the temperature rises the hydrogen bonds in the liquid state begin to dissolve. Water has the highest known vaporization heat. The heat consumed or released when matter disintegrates, changing state from liquid to gas at a constant temperature is referred to as latent heat of vaporization. The heat of vaporization of water is the most elevated known. The heat of vaporization is defined as the amount of heat required to convert one gramme of a fluid into a fume without causing the fluid's temperature to change. Latent is derived from the Latin word latere, which intends to lie covered up or hid. Latent heat is the additional heat required to change the state of a substance from solid to fluid at its softening point or from fluid to gas at its breaking point after it has reached both temperatures. 
When heat is applied to water, molecules begin to degenerate and vaporize. Because the surface molecules will be cooled, it takes more kinetic energy to evaporate water which has the greatest heat of vaporization. When a liquid inside a sealed jar is heated, it cannot escape by evaporating or becoming gaseous. At this point, the vapor is said to be saturated, and the pressure associated with it is known as saturated vapor pressure. This method is repeated until the quantity of molecules equals the number of molecules in the liquid state. Daily Life examples of Vaporization
Factors affecting the Vaporization
Because increasing an object's surface area exposes more particles to changes in temperature, the surface area is directly proportional to the rate of vaporization.
As we reduce the pressure, the particles obtain the kinetic energy needed to escape from the container's surface. As a result, the pressure is indirectly proportional to the rate of vaporization.
As the temperature rises, so does the kinetic energy required by the particles to escape from the container's surface. As a result, the force of interaction between particles decreases. As a result, the temperature is directly proportionate to the rate of Vaporization.
Wind speed is directly proportional to vaporization because as wind speed increases, more particles are driven away by the wind. Difference Between Evaporation and VaporizationEvaporation and Vaporization convert a liquid substance into its gaseous phase. The main difference between Evaporation and Vaporization is that Evaporation tends to happen at temperatures lower than a liquid's boiling point. whereas vaporization will occur at the liquid's boiling point. Evaporation also occurs when the vapor pressure of a liquid is lower than the external pressure enclosing the liquid. On the other hand, Vaporization happens when the water content of a liquid equals the external pressure. 
Although both Evaporation and boiling involve the transformation of liquid into gas, Evaporation only refers to the surface level of the liquid becoming a gas. In contrast, the internal vaporization pressure of the liquid remains low. When a substance boils, the vaporization pressure rises rapidly, causing the surface to evaporate at the same rate as the rest of the liquid. The presence of bubbles, which only occur during the boiling phase and not during the evaporation process, is an indication of boiling.
Evaporation only changes the matter's state from liquid to vapor, so vaporization changes the phase of matter from liquid to gas. 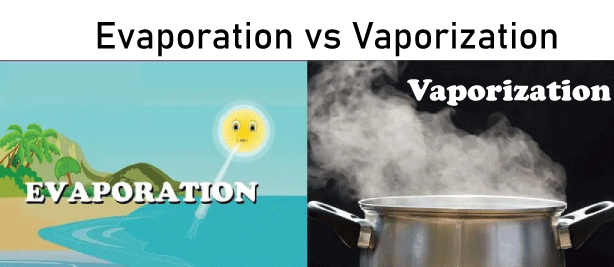
In this section, we will look at the differences between vaporization and evaporation and why they're not the same thing. Many people often need clarification on these two processes, and these terms are frequently misunderstood. They appear to have similar meanings, but they do not. Furthermore, these processes differ only marginally when viewed at the molecular level. They both occur on different surfaces, at different times, and at different temperatures. However, when you closely examine these two processes, you will notice several distinct characteristics.
The ConclusionThe transformation of an element or compound from a solid or liquid phase to a gas phase is known as vaporization. In contrast, Evaporation is a type of Vaporization in which the transition from a liquid phase to a gas phase occurs on the surface and below the boiling temperature at a given pressure. Evaporation and vaporization change the phase or state of the matter from solid to gas. Vaporization can occur via boiling, sublimation, or Evaporation, meanwhile, Evaporation occurs by converting matter from a liquid to a gas with the ideal temperature, humidity, and air movement.
Next TopicDifference between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









