Difference Between Galvanic Cells and Electrolytic CellsIn the Galvanic Cell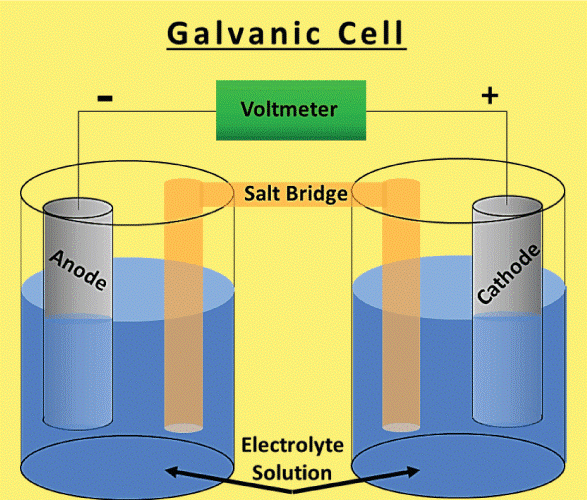
By mixing two different metals in a liquid solution, a galvanic cell is created. An electrical current is produced when the metals and electrolytes react. Electrical current may power devices, and metals can also be made using it. The Electrolytic Cell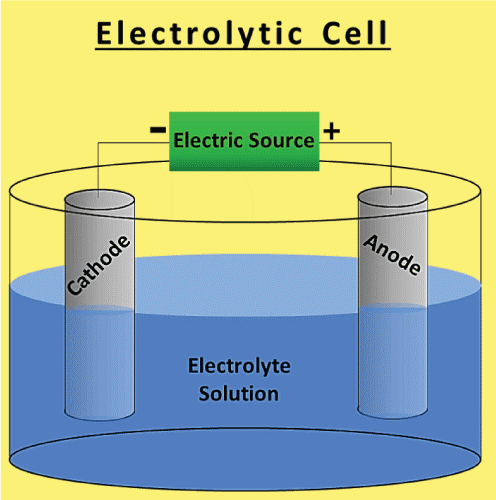
An electrolytic cell is one that conducts chemical processes using electricity. When an electric current passes through a liquid or solid electrolyte in an electrolytic cell, chemical processes happen.These processes may produce metals, salts, or other compounds. Differences Between Electrolytic and Galvanic cellThere are several differences to be made between galvanic and electrolytic cells. Here are some of them in a table. 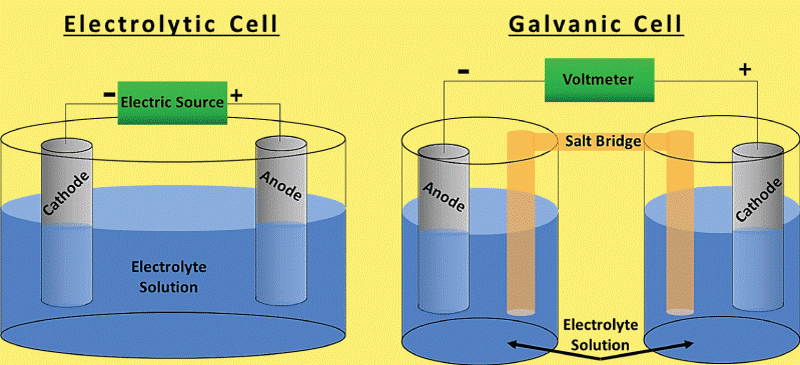
How Galvanic Cells Operate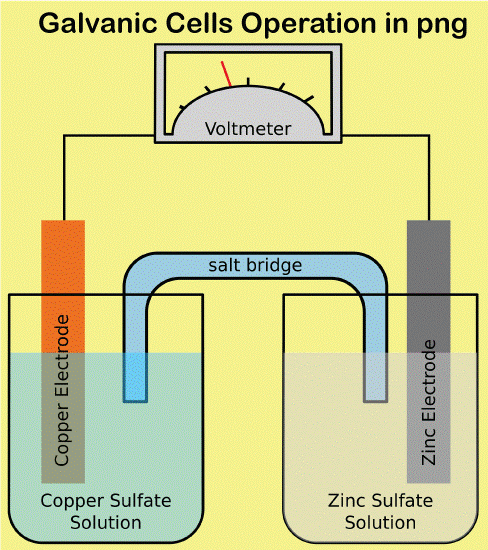
A galvanic cell is created when two different metals come in touch with one another while surrounded by an electrolyte. Electrons can move between the metals with the help of the electrolyte. When an electron flow causes a current, the cell produces electrical energy. The anode and cathode are the two elements that make up a galvanic cell. Anode refers to the metal that loses electrons, and cathode refers to the metal that gains electrons. Typically, the cathode is the less reactive metal, while the anode is the more reactive metal. Between the electrode and cathode is an electrolyte. The electrolyte is a substance that disintegrates in water to produce ions. Ions are atoms or compounds that are electrically charged and have either acquired or removed an electron. The electrolyte acts as a route for the transfer of electrons between the electrode and the cathode. In a galvanic cell, the anode is the negative electrode, and the cathode is the positive electrode. Always choose a more reactive metal for the anode than the cathode. A less reactive metal than the anode is always used to make the cathode.The battery powers the cell by supplying energy to the anode and cathode through its connections. The battery's anode and cathode are compelled to exchange electrons. When an electron flow produces a current, the cell produces electricity. How Electrolytic Cells Operate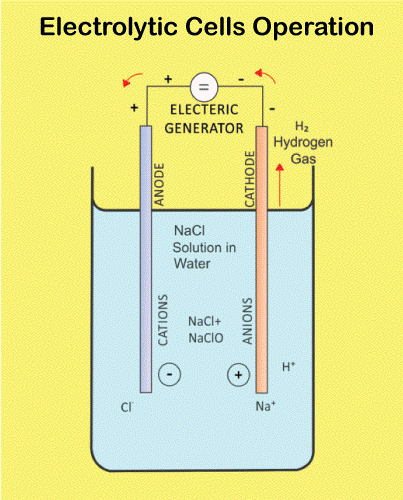
A current is transmitted through an electrolyte that is either liquid or molten in an electrolytic cell. As a consequence, one or more molecules are oxidized at the anode, and one or more molecules are reduced at the cathode. One way to represent this is with the equation below: Oxidation reaction: anode 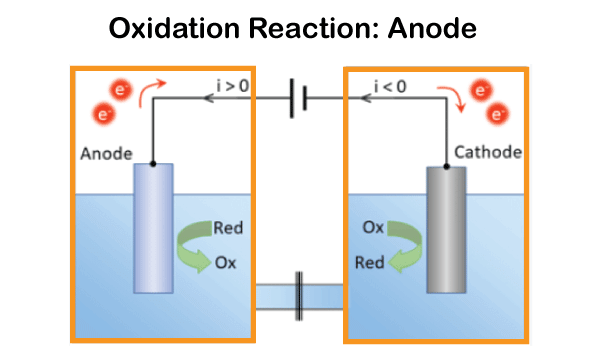
Reduction reaction: cathode 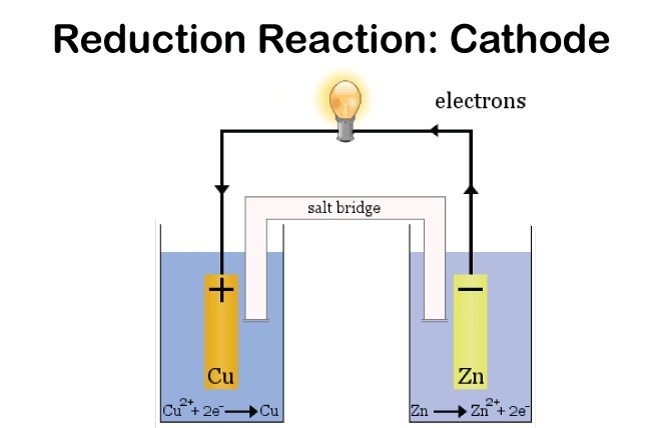
In an electrolytic cell, the anode is the electrode where oxidation occurs, whereas the cathode is the electrode where reduction occurs. Usually, the anode is made of a more reactive metal than the oxidized species. Generally, the cathode is made of a less reactive metal than the species being reduced. ConclusionBecause of the differences between galvanic and electrolytic cells that were previously covered, a galvanic cell generates an electric current in addition to a chemical reaction that naturally occurs inside of it. In contrast, an electrolytic cell accomplishes the exact opposite. Itindicates that an external electric current is used to initiate the chemical reaction. Commonly Asked Questions about Galvanic Cells and Electrolytic CellsQuestion 1: What is a galvanic cell's purpose? Answer: A galvanic cell is a particular form of an electrochemical cell. Via the transfer of electrons in a redox process, it provides electrical current. The galvanic cell is an example of how essential chemical interactions between a few components may be used to generate energy. Question 2: How are voltaic cells made? Answer: The primary cell, also known as the voltaic cell, comprises two copper and zinc electrodes that are submerged in a glass container filled with a diluted sulfuric acid solution. Current flows from copper to zinc outside the cell and from zinc to copper inside the cell when the two electrodes are connected outwardly by a wire. Question 3: What is an excellent way to describe a galvanic cell? Answer: Zinc and copper plates are dissolved in diluted sulfuric acid to create a primary voltaic cell. Voltaic cells are electrochemical devices that generate energy through a chemical process. Processes for oxidation and reduction are divided into half-cells. Question 4: What steps are involved in electrolysis? Answer: By running an electric current through a substance, an element can be separated from a compound using electrolysis. For instance, oxygen gas is produced at the anode, and hydrogen gas is made at the cathode when water is electrolyzed. Question 5: An electrochemical cell is what? Answer: A device that produces a chemical reaction using electricity is called an electrochemical cell.
Next Topicdifference-between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









