Difference Between Ionic and Covalent CompoundIntroduction:Someone rightly says, "The meeting of two personalities is like the contact of two chemical substances. If there is any reaction, both are transformed. The basis for this quote is that it is easy to get a thousand elements but hard to get a useful compound. The discovery of several types of compounds is one of the most notable aspects of today's world. The world is changing at a very rapid phase, and in this changing world, technology plays a very important role. Technology makes everything very easy to do. With the help of technology, anyone can discover anything, and it benefits humanity. Out of the developing technologies, the most notable development which has shaken the world is the discovery of various types of compounds. Day by day use of different types of compounds is increasing, and compounds are the backbone of several chemical industries. ve charges, Ionic compounds are good conductors of electricity. Covalent Compounds are bad conductors of electricity.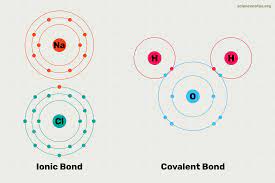
The role of chemicals is important from ancient times, even in earlier times, different types of chemicals were obtained with the help of traditional knowledge. They are exported and imported as per the needs of society. They are Major items in the trade basket. From history, we know that the earliest civilizations engaged in trade; they used to export and import large and deficient items, respectively. Civilizations try to develop several types of chemicals that support society's advancement. The basis of chemicals is nature, and nature provides all the necessary minerals essential for society's development. Nature helps humanity by offering several important compounds necessary for the nation's development. Due to lifestyle changes, the demand for numerous types of chemicals is increasing daily. Due to the invention of certain technologies, discovering several types of chemicals and their compounds is easy. Because of such invention, the needs of future generations are fulfilled. Due to technological advancements, several types of essential chemicals and their compounds are easily obtained, which are tough to acquire at any point in time. The invention of several tools and machinery that helps extract chemicals is a notable development in human history. Numerous types of minerals are obtained from the earth's crust with different physical and chemical characteristics. Scientists developed numerous types of chemicals using these physical and chemical characteristics. Scientists arranged these chemicals in the periodic table for better understanding. They are grouped into several periods in the periodic table for better understanding. The development of chemistry as a separate subject is the biggest achievement in human history. Nowadays, everything is directly or indirectly linked to each other; symbiotic relationships exist between human beings and nature. Almost all fields frame policies and design their products by focusing on these symbiotic relationships. Chemistry is the subject that deals with every aspect of chemicals and their compounds. Under chemistry, scientists try to understand the composition of chemicals, their behavior with other compounds, etc. The basic difference between different types of compounds is the type of bond they form. Generally, the two most outstanding bonds are covalent and ionic compounds. On this basis, there are two types of compounds that are ionic and covalent. This article will discuss ionic and covalent bonds with their characteristics and differentiation. What are Compounds?A compound is a chemical substance of many identical molecules, containing atoms that form more than one chemical element held together by chemical bonds. If any molecule consists atom of a single element, then it is not a compound. With the help of chemical reactions, a compound can be transformed into a different substance. And this involves interactions with other substances. Under this process, bonds between atoms may be broken, and new bonds may be formed. Generally, there are four types of compounds; they differentiate from each other by how constituent atom is bonded. For instance, Molecular compounds are held together by covalent bonds. On the other hand, ionic compounds are held together by ionic bonds. Metallic bonds used in intermetallic compounds and coordination complexes are held together by coordinate covalent bonds. Types of Compounds:In the above paragraphs, we already discussed the compounds. Compounds are substances formed by the union of two or more elements. Compounds are one of the most notable elements in the branch of chemistry. As it provides different types of minerals that benefit humanity. Let us discuss some of the types of compounds- 1. Ionic Compounds:In chemistry, ionic compounds are chemical compounds formed by the association of ions, held together by the electrostatic force of attraction. The force of attraction between the ions of the ionic compound is known as ionic bonding. Ionic compounds are neutral because they consist of both types of ions, which means ionic compounds consist of positively charged ions, known as cations, and negatively charged ions, known as anions. The ionic compounds are simple ions, such as sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species, such as the ammonium and carbonate ions in ammonium carbonate. In the solid state, ionic compounds usually form crystalline structures. Ions in ionic compounds are part of a continuous three-dimensional network because they have multiple nearest neighbors and are not part of molecules. Those ionic compounds that contain basic ions hydroxide (OH−) or oxide (O2−) are known as bases. Ionic compounds without ions are known as salts and acid-base reactions from them. There are numerous ways to produce ions; their constituent ions can produce them after evaporation of their solvent, precipitation, freezing, and a solid-state reaction. Also, the electron transfer reaction of reactive metals with reactive nonmetals, such as halogen gases, can produce ionic compounds. The physical characteristics of ionic compounds are they are hard and brittle with high melting and boiling points. Ionic compounds in the solid state are bad conductors of electricity and insulating in nature. But when the state of ionic compounds is changed from solid to liquid, they are good conductors of electricity and conduct in nature. The reason behind conducting nature is that the ions present in ionic compounds are mobilized. Ionic compounds are one of the essential Compounds in the branch of chemistry as it helps in the formation of several types of chemicals that have usage in several fields of chemistry. 2. Covalent Compounds:Covalent Compounds are other types of compounds formed due to forming a covalent bond between the elements. Covalent bonds are a type of bond that is formed between the compounds after sharing of electrons to form electron pairs between different atoms. The electrons involved in the pair formation are known as shared or bonding pairs of electrons. Covalent bonding is the stable balance between the atoms due to the attractive and repulsive force of attraction. For several molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. Covalent bonding is more important in organic chemistry than iconic bonding. Numerous other types of bonding occur between the covalent interaction: σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds, and three-center four-electron bonds. In 1939 covalent bond was a term introduced in the branch of chemistry. For instance, when we see molecule H2, the hydrogen atoms share two electrons with the help of covalent bonding. Similar electronegative atoms have the greatest Covalency. And because of that, the covalent bonds do not necessarily require that the two atoms be of the same elements. The only requirement is that they are of comparable electronegativity. Covalent bonding that entails sharing electrons over more than two atoms is said to be delocalized. Other types of bonds formed between the different Compounds, for example, Metallic bonds used in intermetallic compounds and coordination complexes, are held together by coordinate covalent bonds. In the field of chemistry, numerous types of compounds are present; they are formed by one of the bonds discussed above. Properties of Compounds:In the above paragraphs, we already discussed compounds and their types. There are several types of compounds found in the branch of chemistry, but what differentiates between them is their properties. Every compound in the branch of chemistry has different properties; for instance, every compound has different melting points, etc. Similarly, numerous properties contribute to the formation of types of compounds. Let us discuss some of the most notable properties of compounds - Ionic Compound Properties:Let us start with the properties of ionic compounds. Some of the properties are -
Physical properties of ionic compounds- Ionic compounds have a strong force of attraction between negative and positive ions; ionic compounds are solid and hard to break. They required a large amount of force to break into pieces. When a huge amount of pressure is applied to the ionic compound components, they are broken into small pieces. Hence due to this property, ionic compounds are considered as brittle. Another remarkable property of ionic compounds is that they have high melting and boiling points; the reason behind high melting and boiling points is they have a high range of electrostatic force of attraction between the ions of compounds. Different ionic compounds have different solubility; generally, they are insoluble in the solid state, but in the molten state, they are soluble. Polar solvents such as water are soluble, whereas solubility tends to decrease in non-polar solvents such as petrol, gasoline, etc. Ionic compounds are considered bad conductors of electricity; they do not conduct electricity in a solid state but are good conductors in a molten state. Electricity involves the flow of charge from one point to another. In solid-state ionic compounds, ions are not free, and their movements are not possible; because of that, they don't conduct electricity. But in a molten state, ionic compounds conduct electricity; the reason behind this is that electrostatic forces of attraction between the ions are overcome by the heat released. Covalent Compounds Properties:As compared to ionic compounds, covalent Compounds have some different types of properties.
Covalent Compounds are insoluble in water; the reason behind this is water molecules are not neutral. It means that compounds have slight negative charges on the oxygen atom and slight positive charges on the hydrogen atoms. We know that covalent compounds are made up of neutral molecules or molecules with slight charges and hence are not attracted to water molecules strongly. In, Summary let's discuss some of the most notable differences between these two compounds. The main differences between these two compounds are - 1. Formation of Compounds: The formation of any compound is the main thing in the branch of chemistry. Any compound needs a special process for its preparation. Different types of processes form both Ionic and Covalent Compounds. As we already know, ionic compounds are formed with the help of electron transfer, and covalent bonds are formed due to sharing electrons. In simple terms, Ionic Compounds are formed with the transfer of positively and negatively charged electrons. On the other hand, covalent Compounds are formed by sharing of electrons. 2. Formation of bonding: The bonding formation is another main thing that creates the difference between compounds. The formation of bonding takes place between different types of elements. For instance, in ionic compounds, bonding occurs between metals and nonmetals. And in the case of covalent compounds, bonding formation takes place between nonmetals. 3. Solubility of Compounds: Solubility is one of the most remarkable properties of compounds. Numerous types of compounds are soluble and insoluble in water and other fluid substances. Regarding solubility, Ionic Compounds are more soluble in water than covalent Compounds. 4. The shape of Compounds: The shape of compounds is an essential property that helps to create a difference between different types of compounds. The formation of shapes is very common in ionic compounds. Ionic compounds have definite shapes; conversely, covalent Compounds have no shapes. 5. The conductivity of Compounds: The conductivity of Compounds is a very important property of compounds. Due to the presence of positive and negati
Conclusion:Both compounds are essential in the branch of chemistry. They are related to different concepts of chemistry. Due to this type of compound, scientists can produce some important substances that have the potential to benefit humanity. Both compounds are interrelated each other, and they are very important in the field of chemistry.
Next TopicDifference Between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









