Difference between Nucleotide and NucleosideNucleotide DefinitionNucleotides, a type of chemical molecule, are the building blocks of DNA and RNA. They also participate in cell signalling, enzyme activities, and metabolic processes. A nucleotide is made up of a phosphate group, a sugar with five carbons, and a nitrogenous base. The four nitrogenous bases contained in DNA are adenine, cytosine, guanine, and thymine. RNA contains uracil instead of thymine. The genetic material in all known living creatures is composed of a chain of nucleotides. They also perform a variety of functions in addition to storing genetic information, including acting as molecules that move energy and messages. A codon is a group of three nucleotides found in DNA that instructs the cell's proteins to link a particular protein to a group of nucleotides specified by the remainder of the DNA. Even where to halt and start the process is specified to the machinery by certain codons. DNA information is transformed into the language of proteins through a process called DNA translation. Once appropriately folded, this chain of amino acids can perform one of the cell's many functions. Nucleotide StructureAlthough the structure of a nucleotide is straightforward, the structure it can take when joined is complicated. Here is a picture of DNA. Two strands make up this molecule, which wraps around itself to generate hydrogen bonds in the centre for support. Because of the distinct structures of each of the nucleotides, this creation is possible. Examples of NucleotideAdenine Adenine is a member of the purine family, one of the two families of nitrogenous bases. The purine have nucleotide's double-ringed structure. In DNA, adenine and thymine connect one another. In RNA, adenine and uracil join. The nucleotide adenine functions as the base in adenosine triphosphate. From there, three phosphate groups can be connected. As a result, a lot of energy may be stored in the bonds. The same property that makes the sugar-phosphate backbone so strong also applies to the bonds in ATP. It pairs with special enzymes that have evolved to liberate the energy, which can then be transferred to other reactions and molecules. Guanine Guanine is a double-ringed purine nucleotide, just like adenine. It connects to cytosine to form a bond in both DNA and RNA. Three hydrogen bonds bind guanine and cytosine together. This makes the cytosine-guanine link slightly stronger than the thymine-adenine bond, which only produces two hydrogen bonds. Cytosine The other category of nucleotides is pyrimidines. A pyrimidine nucleotide with only one ring in its structure, cytosine. Cytosine and guanine join in both DNA and RNA. The two link together in a powerful combination with the nucleotide guanine... Thymine Thymine, like the nucleotide cytosine, is a pyrimidine nucleotide with a single ring. In DNA, it binds to adenine. RNA does not contain thymine. They are the weaker pair in DNA since there are only two hydrogen bonds they can make with adenine. Uracil A pyrimidine is also uracil. Everywhere that a thymine would typically be located, uracil is put instead during the transcription of DNA into RNA. Though uracil has certain distinguishing benefits and drawbacks, the exact cause for this is unclear. Most organisms do not use uracil because it degrades quickly into cytosine and has a short life span. However, as RNA is a short-lived molecule, uracil is favoured as a nucleotide in RNA. Nucleotide FunctionA nucleotide can have a variety of purposes in addition to being the fundamental building block of all living organisms' genetic material. The basic energy molecule of the cell, adenosine triphosphate (ATP), is an illustration of a molecule in which a nucleotide can perform the function of a base. They can also be found in coenzymes like NAD and NADP, which are made from ADP and used in a variety of chemical reactions that affect metabolism. Another molecule that contains a nucleotide is called cyclic AMP (cAMP), a messenger molecule that is essential for many processes like controlling metabolism and sending chemical messages to cells. Nucleotides are the building blocks of life and can be combined to form a vast range of chemicals. Nucleoside DefinitionA pentose sugar molecule joined to a nitrogenous base or glucosamines is known as a nucleoside. A nucleotide without a phosphate group linked to it is another way to describe a nucleoside. D-ribose sugar is present in RNA nucleosides as well as 2'-deoxy-D-ribose sugar in DNA nucleosides. The key distinction is in the second position of the pentose structure, where there is no alcohol group, oxy group, or -OH group in the case of 2'-deoxy-ribose, thus the name. In the instance of D-ribose pentose, the -OH group is present at the second position. The pentoses are present in both forms in their tight five-membered ring structure known as the -furanose form. Types of NucleosidesBased on the existence of the compound's nitrogen base, the nucleosides can be separated into two categories.
1. Nucleosides of purines Adenine and Guanine, two nitrogenous bases, make up these nucleosides. Adenine and Guanine are the nucleosides found in RNA. The nucleosides in DNA are deoxyadenosine and deoxyguanosine. 2. Nucleosides of pyrimidine Thymine, Cytosine, and Uracil are the three nitrogenous bases that make up these nucleotides. Cytidine and Uridine are the nucleosides found in RNA. The nucleosides in DNA are either deoxycytidine and thymidine or deoxythymidine. Structure of Nucleoside
Nitrogenous base
Functions of Nucleoside
Difference between Nucleoside and Nucleotide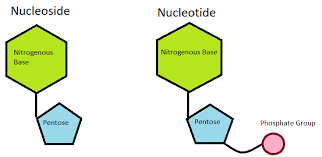
Now that we have understood what exactly Nucleotides and Nucleosides are, let's look at this table which highlights all the key differences between the two:
Next TopicDifference between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










