Difference between Quality control and Quality AssuranceQuality ControlA company's efforts to maintain or raise product quality are achieved through the quality control (QC) process. The establishment of a culture of perfection among management and staff is necessary for quality control. This is accomplished by staff training, the development of product quality norms, and product testing to look for statistically significant variances. The implementation of well specified controls is a crucial component of quality control. These measures aid in standardising both production and responses to problems with quality. Specifying which production tasks are to be carried out by which employees limits the margin for error and lowers the likelihood that workers will be assigned to duties for which they are not properly trained. Understanding quality controlQuality control is the process of evaluating units to determine if they satisfy the specifications for the final product. The aim of the testing is to pinpoint any areas where the production process needs to be improved. Effective quality control enables businesses to better meet consumer demands for enhanced products. Why is QC required?A company may also be liable for any issues or mishaps that arise from using defective items if it sells them. Quality control inspectors make sure that defective or unsafe goods are found, and their causes are remedied. How does it work?Mostly, quality testing is part of every stage of a manufacturing or business process. Starting with testing raw materials, finished products, and samples taken from the production line is a common practise for employees. Testing at various stages of production can assist pinpoint the location of a production issue and the corrective actions needed to stop it from happening again. QC is different by industryThe type of product or industry heavily influences the quality control practises used in a corporation. For instance, quality control in the production of food and drugs entails making sure the product will not make a customer sick, therefore the business tests samples from the production line for chemicals and microorganisms. Quality assurance and control are crucial in the production of aeroplanes. To establishing proof that everything is finished in accordance with very tight requirements, manufacturers are expected to track, document, inspect, and reinspect all parts and phases of a build. The goal of quality control in the automobile industry is to ensure that parts adhere to tolerances and requirements. QC makes certain that motors, transmissions, and other mechanical components function properly, effectively, safely, and according to plan. When checking the quality of electronics, stress tests and electrical flow metres may be used. Types of quality control methodsCommunicate and keep track of inspections and problems, quality control employs a number of different techniques. A quality control chart, for instance, is a visual representation of whether sampled items or processes are following their intended specifications-and, if not, how far they deviate from those criteria. When a single chart analyses just one product aspect, it is known as a univariate chart. A multivariate chart is one that analyses variations in a number of product parameters. Businesses may identify how many errors they make per manufacturing unit and what kinds of defects are happening by tracking variations. There are few samples of some of the techniques employed in this: X-Bar chart Products are evaluated for the specified properties the chart is tracking using a random selection process. Popular quality control charts, such as the X-Bar Chart, measure how much variation in the tested feature is tolerable by plotting it along the graph's y-axis. The tested samples are tracked on the x-axis. You can tell whether problems are happening randomly or consistently by examining the variance pattern on this chart. Taguchi method An additional strategy is the Taguchi Method of quality control, which emphasises how research and development, product design, and product development collaborate to lessen the risk of product flaws and failures. The Taguchi Method seeks to prevent production deviations from happening by prioritising design above the manufacturing process in quality control. 100% inspection method As part of the quality control process, all product components are examined and evaluated using the 100% inspection method. Rule out product faults, this kind of quality control is carried out. This approach is frequently used to assess precious metals and products. The 100% inspection approach requires information on the manufacturing process and inventory analysis software. Quality AssuranceQuality assurance (QA) is a systematic process for determining if a good or service complies with predetermined specifications. A quality assurance system increases work processes, efficiency, client confidence, and a company's credibility, allowing a business to compete more successfully with other companies. The International Organization for Standardization (ISO), which is a major influence behind QA procedures and process mapping, is responsible for implementing QA. QA and the worldwide ISO 9000 standard are frequently combined. The manufacturing sector introduced the idea of quality assurance as a structured technique, and it has subsequently expanded to most industries, including software development. Importance of quality assuranceA company can use quality assurance to provide goods and services that meet the needs, requirements, and standards of its customers. It creates premium product offers that promote consumer loyalty and trust. The guidelines and procedures of a quality assurance programme help to stop product faults before they happen. Quality assurance methodsQuality assurance uses these three methods:
QA standardsThe ISO standards must change to remain applicable to today's enterprises, much as QA standards have evolved and been updated throughout time. Stronger customer focus, top management practises and how they might alter an organisation and keeping up with ongoing improvements are among the recommendations in ISO 9001:2015. Along with other general updates to ISO 9001, ISO 9001:2015 also incorporates structural updates and more data for risk-based decision-making. Quality assurance in softwareA methodical technique is used by software quality assurance (SQA) to spot trends and determine the best ways to improve development procedures. Unintended effects might result from finding and resolving coding problems; it is possible to solve one issue while also breaking other features and functions. SQA has grown in importance for developers to stop mistakes in their tracks and cut down on costs and development time. An upgrade to software can damage other features and result in defects, sometimes known as bugs, even with SQA protocols in place. There are several different SQA tactics. For instance, the Capability Maturity Model Integration (CMMI) is a SQA model with a performance enhancement focus. CMMI ranks the organisational areas' maturity levels and identifies optimizations that can be used to improvement. The spectrum of rank levels includes chaotic to perfectly ideal. Over time, SQA-based software development techniques, including Waterfall, Agile, and Scrum, have emerged. Every development process aims to maximise labour productivity. The conventional, linear method of software development is called waterfall. It is a step-by-step process that usually entails gathering requirements, formalising a design, putting code into place, evaluating the code, fixing any issues, and then releasing it. Alternative development techniques were created since it is frequently thought to be overly sluggish. Agile is a team-focused approach to software development where each stage of the project is treated as a sprint. Agile software development is very adaptable, but it lacks predictability because the project's scope might alter at any time. Developers are divided into teams to perform specific tasks, and each job is divided into many sprints in Scrum, which combines both procedures. Setting standards targets is the first step in implementing a QA system. Think about each strategy's benefits and trade-offs, such as maximising effectiveness, cutting costs, or minimising mistakes. Support the QA system and create quality standards, management must be eager to modify processes and collaborate. Key difference between quality assurance and quality control
Difference between quality control and quality assurance
Quality Assurance vs. Quality Control: 5 differences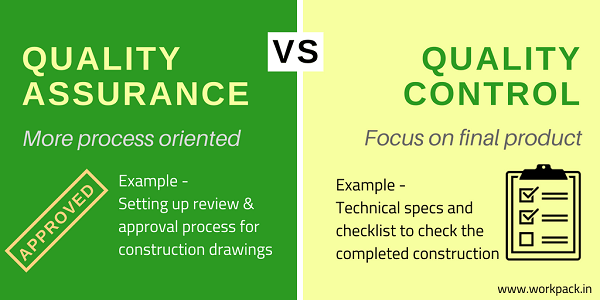
One need to understand how the two processes interact to improve quality within the organisation and minimise corrective actions to better comprehend the differences between quality assurance and quality control. 1. Proactive (QA) vs. Reactive (QC)Proactive quality control is essential. Through process design, it seeks to stop faults in their tracks before they start. QC is reactive and serves to spot product quality flaws after they have occurred. Designing processes for QA includes things like creating ISO 9000-compliant standard operating procedures (SOPs) and documenting them. Every time processes are adhered to, a safe, effective product should be the result. QC entails evaluating products to make sure they adhere to safety and efficacy requirements. If QC testing reveals quality problems, remedial action should be taken to stop an unsafe product from being delivered and disseminated. In a perfect world, QC concerns would also prompt a QA evaluation. When test findings are non-conforming, corrective and preventative action (CAPA) should be taken to investigate the source of the problem and improve procedures to make sure it does not happen again. 2. Process (QA) vs. Product (QC)Process-driven QA places a strong emphasis on preventing quality issues. Finding manufacturing-related quality issues that could lower consumer satisfaction is the aim of quality control. The concept of acts vs results can also be used to explain this conflict. QA is concerned with the production procedures, whereas QC is focused on the final product. Below are a few illustrations of each kind of activity. QA processes: Documentation, audits, supplier management, training for employees, change management, and investigation protocols. QC processes: Batch inspection, product sampling, validation testing, lab testing, and software testing are a few examples. 3. System (QA) vs. Parts (QC)Systems of quality assurance and control are employed to protect the highest standards of quality. Systems for quality control measure several components, including the system's outputs. The focus of QC efforts may also be on components required to make the finished product, including raw materials obtained from a supplier. Ensure that inputs are consistently safe and effective, the QA system for quality management may specify a number of actions, such as auditing suppliers and batch sampling raw materials. 4. Creation (QA) vs. Verification (QC)A strategy for producing high-quality products is the end consequence of QA operations. Standards must be established for product development, production, packaging, distribution, marketing, and sales. QC entails checking products after they are manufactured and before they are distributed or ensuring their safety and effectiveness. 5. Entire team (QA) vs. Dedicated personnel (QC)The entire team participates in quality assurance tasks. Following SOPs, each employee of a life sciences business is accountable for QA tasks. While the leadership team and the quality unit are often in charge of the quality management system (QMS), QA activities entail uniform standards for training, documentation, and review for the entire workforce. QC is the duty of specific employees inside the firm, whose responsibilities include adhering to SOPs for product testing. Based on defined methods for product testing and process validation, QC staff adhere to SOPs for quality control and record their findings.
Next TopicDifference between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










