Difference Between Single Displacement Reaction and Double Displacement ReactionIntroductionWhen another partially replaces one reaction, this is called a displacement reaction. The terms "displacement response" and "replacement reaction" are similar. In displacement reactions, there are two categories: 
Single Displacement Reaction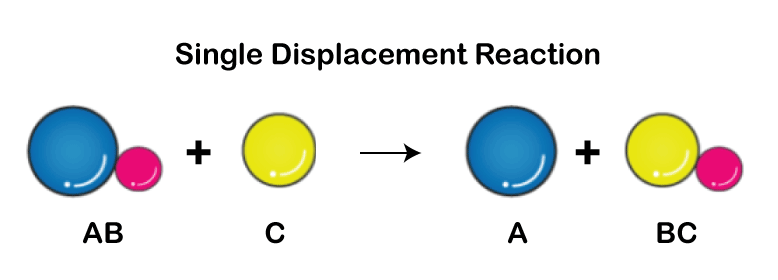
A single displacement reaction, often called a single replacement reaction, happens when one element in a compound is replaced by another, expulsing an ion or other part from the molecule. Double Displacement Reaction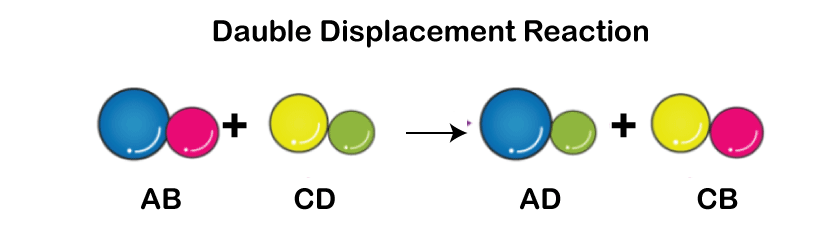
A double displacement reaction occurs when two ionic compound components are transferred, forming two new elements. Distinctions between a Single Displacement Reaction and a Double Displacement Reaction.
Single displacement reaction examples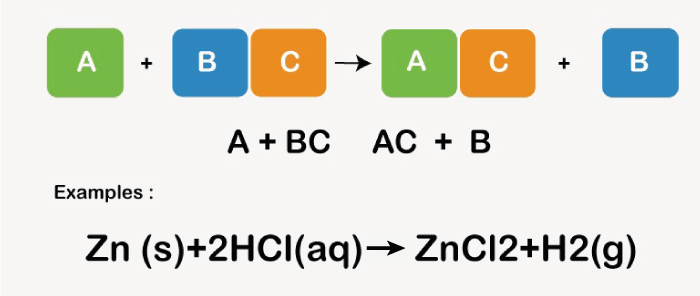
The reaction of zinc metal with hydrochloric acid to produce zinc chloride and hydrogen gas is an illustration of a single displacement reaction. Zn(s) + 2HCl(aq) → ZnC (aq) + (g) Because zinc is more reactive than hydrogen, there was just one displacement in this instance. A Double Displacement Reaction example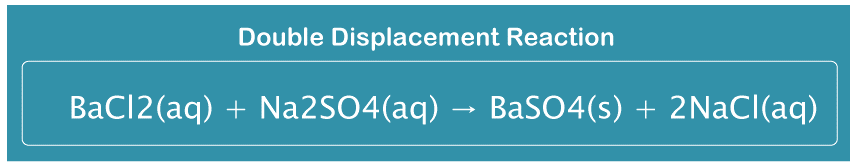
A white barium sulfate precipitate occurs fast when sodium sulfate and a barium chloride solution are mixed. BaC (aq) + N S (aq) → Ba (s) + 2NaCl(aq) These reactions are ionic. When the reactants are dissolved in water, ions are produced. A product molecule is produced in the solution as a result of the substitution of sodium and sulphate for barium and chloride. ConclusionIn displacement reactions, a new element takes the place of one of the reactants in a chemical equation. Two components move into two different locations in double displacement reactions. These sorts of reactions are vital to understanding since they can lead to the development of new compounds. Displacement reaction and double displacement reaction frequently asked questionsQuestion 1: Are double replacement acid-base reactions? Answer: In double substitution processes, anions or cations are exchanged between two ionic molecules. When acid and base are the reactants, neutralization reactions take place, and neutralization reactions are typically favorable as long as the response necessitates a solid acid. Question 2: Why don't some double displacement reactions happen? Answer: In a double displacement process, two salts typically exchange ions. Ion exchange is prevented in a solid state by a high ionic force of attraction. Thus, the double displacement reaction is controlled. Question 3: How can you be sure that a double displacement reaction won't occur? Answer: We need to understand the types of ionic compounds that create residues to determine if double-replacement reactions will occur. Solubility rules, which are broad generalizations that indicate which ionic compounds are soluble and which are not (or are not soluble or insoluble), are used for this. Question 4: Fast or sluggish double displacement reaction? Answer: Speed: As just one element changes, displacement reactions are often faster than double displacement reactions. Because two components must shift positions, double displacement reactions are often slower. Reactions: There is just one displacement in a displacement reaction. Question 5: Do all double displacement reactions generate solids? Answer: In an aqueous solution, double-replacement reactions often take place between compounds. A response must produce one of the following: a solid residue, a gas, or a molecular substance like water.
Next Topicdifference-between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









