Difference between paramagnetic and diamagneticThere are three types of magnetic materials, namely ferromagnetic, paramagnetic, and diamagnetic. The behavior of a material or substance under the application of external magnetic fields depicts their category (ferromagnetic, paramagnetic, and diamagnetic). The effect of the applied magnetic field on the atoms of these three magnetic materials is shown in the below image: 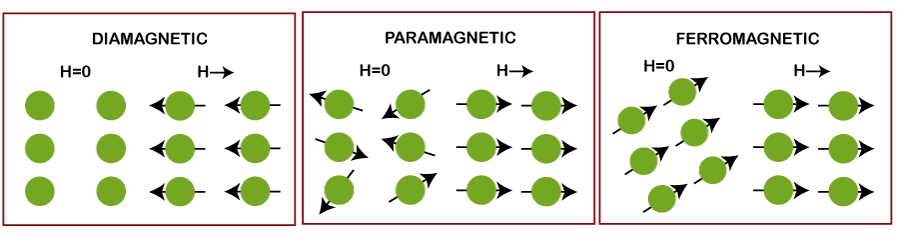
Here, we will discuss the paramagnetic and diamagnetic materials in detail. Paramagnetic materialsThe paramagnetic materials are weakly attracted towards a magnet. It means that the paramagnetic materials are weakly attracted under the effect of any applied magnetic field. Dipole Moment It is also known as a magnetic moment. It defines the magnetic properties of a magnet. Its SI unit is Ampere per meter square (A/m^2). The dipole moment of the paramagnetic materials is permanent. Let's discuss what the permanent dipole moment signifies. The permanent dipole moment of the paramagnetic materials depicts the different electro-negativity (capability of an electron to attract a shared pair of electrons) of two atoms in a molecule. One atom of that molecule becomes more negative by accepting extra electrons, while the other atom becomes positive. The structure of paramagnetic materials is shown below: 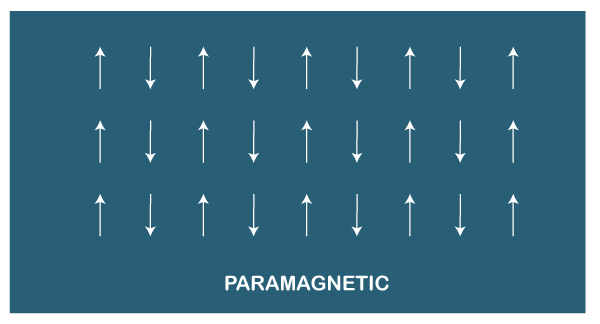
The attractions of the paramagnetic materials towards magnet are weaker than ferromagnetic materials. Hence, the detection of paramagnetic materials requires sensitive magnets or very strong magnets. Unlike ferromagnetic materials, it cannot retain magnetism after the withdrawal of the applied magnetic field. The examples include aluminum, titanium, etc. We can clearly notice the unpaired electrons in the structure shown above. Let's discuss the role of unpaired electrons and how it impacts the magnetic property of the paramagnetic materials. Unpaired electrons mean that an electron occupies the orbit singly rather than in a pair. The two electrons present in the atom now have opposite spin. The presence of these materials causes the electrons to align themselves opposite to each other and attracts. The attraction produced is not strong. The direction of the atom of a paramagnetic material aligns in the same direction as the magnetic field. Hence, the produced attraction is weak. Thus, we can say that the magnetic field lines can pass through the paramagnetic material, as shown below: 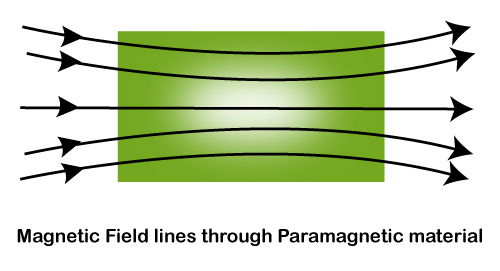
The paramagnetic materials cannot retain magnetism like ferromagnetic materials. Let's discuss why? The spin of the electrons under the applied magnetic field is the opposite. After the removal of the magnetic field, the electrons spin randomly and there exists no net magnetism. Hence, such materials cannot retain any magnetism after the withdrawal of the applied magnetic field. We can also say that the thermal motion after removing the magnetic field results in random spin orientations. Diamagnetic materialsThe structure of diamagnetic materials consists of paired electrons, as shown below: 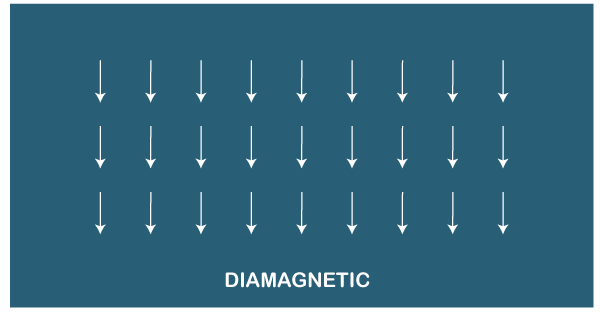
Due to the paired electrons between the atoms, these materials cannot generate their magnetic field. Diamagnetic materials repel or oppose any applied magnetic field. Let's discuss this in detail. The permeability of such material is less than that of a vacuum. If a substance does not possess any paramagnetic or ferromagnetic, it means that it is diamagnetic. Most of the elements in the periodic table are diamagnetic. The electron pairs in the diamagnetic materials are linearly aligned under the application of the applied magnetic field. It means that it repels or opposes the magnetic field. Let's discuss why. The electron pairs in the diamagnetic materials are together, which results in 0 (zero) total spins. The resultant spin produces a small current, which obstructs the applied magnetic field. Hence, the diamagnetic materials weakly repel under the applied magnetic field. Thus, we can say that the magnetic field lines do not pass through the diamagnetic material, as shown below: 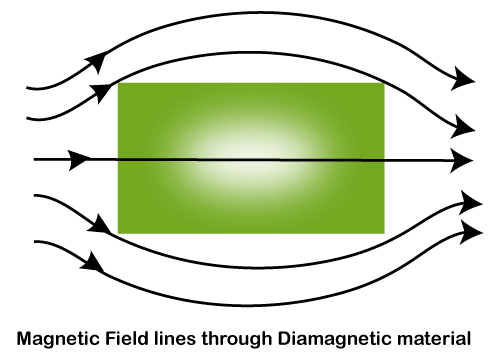
The examples include bismuth, antimony, mercury, etc. Bismuth and graphite (a crystalline form of carbon) are considered the strongest diamagnetic materials. After the removal of the magnetic field, the atoms lose their internal energy. It results in no net dipole moment. Hence, the dipole moment of diamagnetic materials is not permanent. Paramagnetic vs. DiamagneticLet' discuss the difference between paramagnetic and diamagnetic materials.
FAQsFor better understanding, let's consider some frequently asked questions about paramagnetic and diamagnetic. If paramagnetic are weakly attracted, then why ferromagnetic are strongly attracted?We know that paramagnetic materials are weakly attracted and cannot retain magnetism, as discussed above. But, ferromagnetic materials are strongly attracted. Let's discuss the reason behind it. The ferromagnetic materials have some unpaired electrons. When the magnetic field is applied to such materials, the interaction of electrons will align them in the applied magnetic field direction. The magnetic moments of such materials also align themselves parallel in the same direction as the magnetic field. Hence, these materials are strongly attractive. In other magnetic materials, the alignment of dipole moments is in more than one direction. Ferromagnetic is the only material or substance that align it in the same direction as the magnetic field. Why are paramagnetic materials weakly attracted under the applied magnetic field?The unpaired electrons of the paramagnetic materials under the applied magnetic field align themselves opposite to each other. Such opposite spin electrons result in no net magnetic field. But, it acts as a small magnet. Hence, the paramagnetic materials are weakly attracted under the applied magnetic field. What susceptibility of the paramagnetic and diamagnetic signify?Susceptibility of the paramagnetic and diamagnetic materials signifies its ability to be magnetized under the application of an external magnetic field. Why paramagnetic have positive susceptibility?The paramagnetic materials are weakly attracted under the applied magnetic field due to the unpaired electrons that have opposite spin. The positive susceptibility depicts the ability of a material to be magnetized under the external magnetic field. The paramagnetic materials are magnetized in the same direction as the magnetic field. Hence, it has a small positive susceptibility. Why diamagnetic have negative susceptibility?The electron pairs in the diamagnetic materials are together, which results in 0 total spins. The direction of the magnetic field of such materials is in the opposite direction to that of the applied magnetic field. It means that the diamagnetic have small negative susceptibility.
Next TopicDifference between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









