Acids and Bases Definition
Definition of Acids and Bases
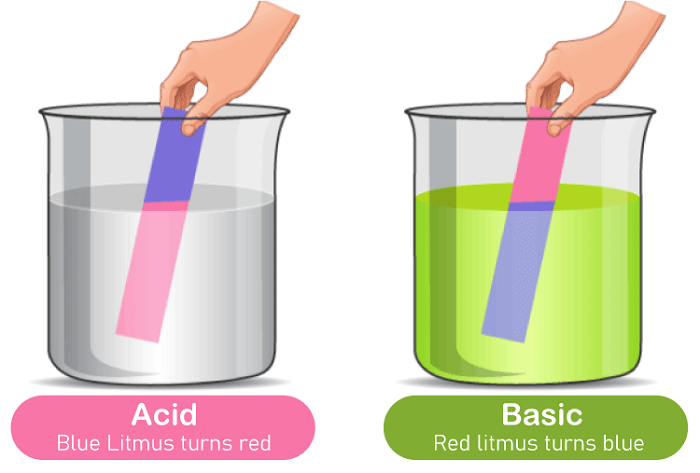
Acids: Any hydrogen-containing material that can donate a proton (hydrogen ion) to another chemical is considered an acid. Often, sour tastes help to identify acidic chemicals. A fundamental definition of an acid is a molecule that can donate an H+ ion and maintain its energetic favorability even after losing H+. Acids are known to turn blue litmus paper crimson.
Base: A base is a molecule or ion that can take up an acid's hydrogen ion. On the other hand, bases have a bitter flavour and a smooth consistency. Alkali is the name for a base that can dissolve in water. Salts are produced when these compounds interact chemically with acids. It is known for bases to make red litmus blue.
Acids and Bases Theories
To define acids and bases, three alternative hypotheses have been proposed. The Arrhenius theory, the Bronsted-Lowry theory, and the Lewis theory of acids and bases are the name of these theories. This subsection offers a brief explanation of each of these theories. Three alternative hypotheses can be used to define acids and bases:-
- According to the Arrhenius theory of acids and bases, an acid creates H+ ions in a solution, whereas a base creates an OH- ion in its solution.
- A base is a proton acceptor, whereas an acid is a proton donor, according to the Bronsted-Lowry theory.
- Finally, the Lewis definition of acids and bases depicts "acids as electron-pair acceptors and bases as electron-pair donors".
Acids and Bases pH Values
The pH scale (where pH stands for "potential of hydrogen") can be used to determine the numerical value of a substance's degree of acidity or basicity. The pH scale is the most popular and reliable method for determining how basic or acidic a thing is. The pH scale ranges from 0 to 14, with 0 being the most acidic and 14 being the most basic.
Using litmus paper is another approach to determine whether a chemical is basic or acidic. Red and blue litmus papers are the two varieties of litmus paper that can distinguish between acids and bases. Red is the colour that appears on blue litmus paper. Under acidic conditions, blue litmus paper turns red, and under basic or alkaline conditions, red litmus paper turns blue.
Acid and Base Properties
1. Acid properties
- Acids tend to rust.
- They are effective electrical conductors.
- Their pH levels are never greater than 7.
- These chemicals react with metals to form hydrogen gas.
- Acids have a sour flavour.
- Examples are acetic acid [CH3COOH], sulfuric acid [H2SO4], and hydrochloric acid.
2. Bases properties
Certain things, like bad taste, belong to everyone. Also, the bases seem slick. Imagine what slippery soap appears to be. This serves as a basis. Furthermore, because bases are made up of charged particles in the solution, they conduct electricity when submerged in water.
- When touched, they reveal a buttery texture.
- When these compounds are dissolved in water, hydroxide ions (OH- ions) are released.
- The aqueous solutions of bases are effective electrical conductors.
- Bases have pH values that are always higher than 7.
- Bases can turn red litmus paper blue and have an unpleasant taste.
- Examples include calcium hydroxide, milk of magnesia, and sodium hydroxide (NaOH).
3. Neutral substances
The neutral substance does not change the colour of the litmus surface, is neither acidic nor basic, and contains the same number of hydrogen and hydroxyl ions.
- These chemicals do not demonstrate any acidic or basic qualities.
- Their pH values are close to becoming 7.
- The red and blue litmus papers are unaffected by neutral chemicals.
- Pure water has a pH of exactly 7.
- Examples: Water, regular salt (NaCl).
Uses of Acids and Bases
This subsection is a list of the different applications of acids and bases:
1. Acids' uses
- Acetic acid is diluted into vinegar, used in many household processes. Its main application for it is as a food preservative.
- Lemon juice and orange juice both include citric acid as primary ingredients. Moreover, it can be used to preserve food.
- Acid sulphate is frequently used in batteries. This acid is frequently found in the batteries used to start car engines.
- Sulfuric acid and nitric acid are used in the industrial manufacturing of fertilizers, paints, dyes, and explosives.
- A significant component of many soft drinks is phosphoric acid.
2. Bases' Uses
- Sodium hydroxide is used in the production of soap and paper. The production of rayon also makes use of NaOH.
- Bleaching powder is produced using calcium hydroxide, often known as slaked lime or Ca(OH)2.
- Calcium hydroxide is used in the production of dry mixtures for painting and decorating.
- Laxatives frequently contain magnesium hydroxide, popularly known as milk of magnesia. It is also an antacid since it lowers excess acidity in the human stomach.
- In laboratories, ammonium hydroxide is a necessary reagent.
- The use of slaked lime helps neutralize any excess acidity in soils.
Acids and Bases, According to Arrhenius
- Svante August Arrhenius, a Swedish chemist, defined acids as compounds that, when dissolved in water, raise their H+ ion concentration.
- These protons combine with water molecules to create hydronium ions (H3O+).
- Corresponding to this, according to the Arrhenius definition of a base, bases are compounds that, when dissolved in water, raise the amount of OH-
- This hypothesis has the advantage of successfully explaining how acids and bases react to produce salts and water.
- An explanation for how compounds like NO2- and F-, which lack hydroxide ions, generate basic solutions when dissolved in water is a significant flaw in the Arrhenius definitions of acids and bases.
The Acids and Bases Hypothesis of Bronsted Lowry
- According to the Bronsted-Lowry theory, an acid is a proton donor.
- Bronsted acids produces protons, increasing the amount of H+ ions in the solution.
- In contrast, Bronsted bases take protons from the solvent water to produce hydroxide ions.
- The capacity to explain the acidic or basic character of ionic species is a benefit of the Bronsted-Lowry definition of acids and bases.
- This theory's inability to account for the acidic characteristics of chemicals like BF3 and AlCl3, which lack hydrogen, is a critical weakness.
- According to this hypothesis, a base is a proton acceptor (or an H+ ion acceptor).
Conjugate Acids and Bases
- An acid is a substance that can give H+ away, and a base is a substance that can take H+ in, according to the Bronsted-Lowry idea.
- The conjugate acid and base pair is made up of an acid and a base that differ by one proton.
- A conjugate acid is created when a proton is supplied to a base and vice versa. A conjugate base is created when a proton is taken away from an acid.
Acids and Bases, According to Lewis
- According to the Lewis definition, an acid is a species that has an open orbital and can thus accept a pair of electrons.
- A Lewis base is a species capable of serving as an electron-pair donor since it has a single pair of electrons.
- This theory does not define acids and bases in terms of the hydrogen atom.
- Lewis bases are nucleophilic, whereas Lewis acids are electrophilic by nature.
- Lewis acids include Cu2+, BF3, and Fe3+. Lewis bases include F-, NH3, and C2H4 (ethylene).
- A coordinate covalent bond is created when a Lewis acid accepts an electron pair from a Lewis base. The resulting substance is known as a Lewis adduct.
- This notion has the remarkable benefit of allowing for the classification of numerous substances as bases or acids. Yet, it needs to reveal how powerful these acids and bases are.
- One of its drawbacks is this theory's inability to account for acid-base reactions that do not create a coordinating covalent bond.
Difference Between Acids and Bases
- When dissolved in water, any chemical substance that generates a solution with a higher hydrogen ion activity than purified water is considered an acid. A base, however, is an aqueous material that has the capacity to absorb hydrogen ions.
- While bases depend on the concentration of hydroxide ions, acids depend on the concentration of hydronium ions.
- Acids can appear as solid, liquid, or gas. That would taste sour as well. Yet, bases would feel both slick and firm (except for ammonia, which is gaseous). The flavour would be harsh.
- Hydrogen ions (H+) are released when water and acids are combined. In contrast, hydroxide ions (OH-) are released when bases and water are combined.
- Bases turned red litmus blue while acids turned blue litmus red.
Some Facts about Acids and Bases
To help you understand more about acids, bases, and pH, here are ten facts about bases and acids:
- Any liquid that contains water and is aqueous can be categorised as acidic, basic, or neutral. Acids and bases do not apply to oils or other non-aqueous liquids.
- Acids and bases have varied meanings, but acids can take an electron pair or donate a hydrogen ion or a proton in a chemical reaction, whereas bases can do the opposite.
- Strong and weak acids and bases have different properties. In water, a strong acid or base separates into its ions. It is a weak acid or base if the chemical partially dissociates. A base's or an acid's strength has nothing to do with how corrosive they are.
- A solution's acidity or alkalinity (basicity) is measured using the pH scale. The pH scale ranges from 0 to 14, with seven serving as neutral, acids having a pH less than seven, and bases having a pH greater than 7.
- A neutralisation reaction occurs when acids and bases interact with one another. The reaction results in the production of salt and water and brings the pH of the solution closer to neutrality.
- Wetting litmus paper with an unknown substance is a typical way to determine if it is an acid or a base. Paper treated with a lichen extract known as "Litmus paper" will change colour depending on the pH. Acids turn litmus paper red, while bases render litmus paper blue. A neutral chemical won't alter the colour of the paper.
- Both acids and bases carry electricity because they split into ions in water.
- A solution's acidity or baseness can be distinguished by taste and touch, not by simply looking at it. But you shouldn't test chemicals by tasting or touching them because both acids and bases have the potential to be corrosive. Both acids and bases can cause chemical burns. Bases taste bitter and feel slippery or soapy, while acids typically taste sour and feel drying or astringent. Vinegar (weak acetic acid) and baking soda solution (diluted sodium bicarbonate - a base) are two examples of common household acids and bases that you can test.
- In the human organism, acids and bases play crucial roles. For instance, in order to digest food, the stomach secretes HCl or hydrochloric acid. To neutralise stomach acid before it enters the small intestine, the pancreas secretes a liquid high in the base bicarbonate.
Metals and acids, and bases interact. When metals and acids react, hydrogen gas is released. Hydrogen gas can occasionally be generated when a base combines with a metal, such as zinc and sodium hydroxide (NaOH). A double displacement reaction, which can result in metal hydroxide precipitation, is another frequent reaction between a base and a metal.
|

 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now









