Antibody DefinitionOne of the major elements of the immune system, antibodies, is in charge of identifying and combating pathogens like viruses, bacteria, and other impurities. Antibodies are proteins made by the B cell, the subtype of white blood cells, and are often known as immunoglobulins (Ig). Two similar massive chains and two similar light chains make up the Y-shaped arrangement of these proteins. The pathogen can be neutralized by an antibody's binding to an antigen or marked for eradication by other immune system cells. Antibodies can be divided into five basic categories, each with a distinct structure and function. These include IgM, IgG, IgA, IgD, and IgE. 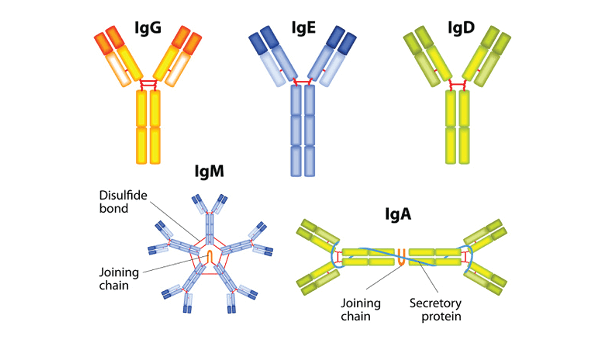
1. IgMImmunoglobulin M, often known as IgM, is a class of antibodies that is the first to be created in response to an infection or invasion by foreign agents like bacteria or viruses. IgM antibodies are crucial in the body's immune response to infectious pathogens and are mostly present in the bloodstream and lymphatic system. IgM antibodies are huge, intricate molecules divided into five monomers and joined by disulphide links. Each monomer has two antigen-binding sites, which allows it to attach to two distinct antigens simultaneously, and comprises two identical heavy chains and two identical light chains. Because they can attach to numerous locations on an antigen simultaneously due to their multivalent nature, IgM antibodies have a high eagerness for antigens. The ability of IgM antibodies to trigger the complement system, a group of blood proteins that cooperate to remove foreign invaders, is one of its key characteristics. IgM antibodies can activate the host immune system when they bind to an antigen by the classical pathway method, eventually destroying the invader. IgM antibodies are particularly good in agglutinating or clumping together pathogens due to their size and multivalent nature, making it simpler for other immune system elements to recognize and eliminate them. IgM antibodies also have a relatively short half-life in the bloodstream compared to other antibody classes, typically lasting for only a few days before being cleared from the body. 2. IgGImmunoglobulin G, a subtype of antibody known as IgG, is a protein found in mammal blood and extracellular fluid. Over 75% of all antibodies that target and neutralize outside material in the human body are IgG antibodies, making them the most prevalent form. The immune system's reaction to infection is strongly affected by IgG antibodies. They can identify and bind to particular pathogens, such as bacteria or viruses, and label them for eradication by other lymphocytes, including phagocytes. IgG antibodies can cross the placenta and give a growing fetus passive immunity. They can also be detected in breast milk and are the reason for immunity in babies. IgG antibodies can be divided into four subclasses (IgG1, IgG2, IgG3, and IgG4), each of which has its characteristics and activities.
IgG antibodies have other uses besides preventing infections, including diagnosis and treatment in medicine. For instance, the availability of IgG antibodies in a person's blood for a particular virus or bacteria suggests that they have previously been exposed to that pathogen. Still, the injection of IgG antibodies created in a lab can give patients with specific diseases passive protection. 3. IgAImmunoglobulin A, often known as IgA, is a kind of antibody largely present in the body's mucous membranes, including those of the digestive and respiratory systems. It is also found in saliva and breast milk. After IgG, it is the second most prevalent antibody in the body. IgA is divided into two subclasses: IgA1 and IgA2. IgA2 is more common in mucosal secretions, whereas IgA1 is more common in serum and the body. IgA can occur in two different forms: monomeric (as a single Y-shaped molecule) and dimeric (as two Y-shaped molecules connected by the J chain). IgA is vital to the immune system's defence of body against infections, especially those that enter through the nasal mucosa. By neutralizing antigens and reducing bacterial attachment to the mucosal surface, it aids in preventing the attachment and invasion of microorganisms. IgA can also stimulate immune cells like neutrophils and macrophages to help the body fight infection. A very common immunodeficiency condition called IgA deficiency occurs when people cannot generate enough IgA. Increased vulnerability to infections, especially those related to respiratory, stomach, and intestine tracts, can result from this. Yet most sufferers of IgA deficiency are silent and do not need to be treated. Overall, IgA is an important component of the body's immune defence, particularly in the mucosal surfaces, where it helps protect against infections and maintain the body's homeostasis. 4. IgDIgD, or Immunoglobulin D, is a class of antibodies found in low concentrations in the blood of mammals, including humans. It is one of the five major classes of antibodies, alongside IgA, IgG, IgM, and IgE. IgD has a structure identical to other immunoglobulins in that it has four protein chains, two of which are heavyweight and two of which are light in weight. The heavy chains have a flexible section that enables the antibody to recognize and bind to particular antigens and a fixed region that establishes the class of the antibody. The light chains also have a variable region, which contributes to the antigen-binding site. IgD is primarily found on the surface of mature B cells, where it acts as a membrane-bound antigen receptor. When an antigen binds to the flexible area of the IgD molecule, it can trigger the activation of the B cell and stimulate the production of other classes of antibodies, such as IgM and IgG. Although IgD is present in the blood, its exact function must be better understood. It is thought to play a role in regulating B cell activation and differentiation and in the immune response to certain types of antigens, such as allergens. Overall, IgD is a relatively understudied antibody, but it is an important component of the immune system's response to infection and disease. 5. IgEIgE (Immunoglobulin E) is a class of antibodies that plays an important role in the immune system's response to parasitic infections and allergies. IgE is the least abundant of the five major classes of immunoglobulins (IgM, IgG, IgA, IgD, and IgE) in the blood. Still, it is present in large quantities on the surface of mast cells and basophils, two types of white blood cells involved in the allergic response. When an allergen (such as pollen, pet dander, or food proteins) enters the body, it can bind to IgE molecules on the surface of mast cells and basophils, causing these cells to release inflammatory chemicals, such as histamine, leukotrienes, and cytokines. This results in the symptoms of an allergic reaction, including sneezing, runny nose, itchy eyes, hives, and in severe cases, anaphylaxis. In addition to its role in allergies, IgE is also important in the immune response to parasitic infections, particularly helminths (worms). IgE antibodies bind to parasites, marking them for destruction by eosinophils, another type of white blood cell. Overall, IgE is an important component of the immune system, although its activity can also contribute to developing allergies and asthma. Understanding the mechanisms underlying IgE-mediated allergic reactions is important for developing new treatments for these conditions. Antibodies are produced in response to exposure to a foreign substance. B cells recognize the antigen and begin to increase, producing large amounts of identical antibodies that can recognize and bind to the antigen. Once the pathogen has been neutralized or destroyed, some B cells become memory cells, which can quickly produce more antibodies if the same antigen is encountered again. This process is called the immune response and is triggered by the presence of antigens. Antibodies have a wide range of medical applications. For example, they are used to diagnose infectious diseases through serological testing, which detects the presence of specific antibodies in the blood. Antibodies can also be used therapeutically to treat cancer and autoimmune disorders. In these cases, monoclonal antibodies are produced in the laboratory and administered to the patient to target and neutralize certain cells or molecules involved in the disease process. ConclusionAntibodies are a vital component of the immune system, playing a key role in recognizing and neutralizing foreign substances. Their ability to bind to specific antigens has various medical applications, from diagnosing infectious diseases to treating cancer. Further research on antibodies will undoubtedly lead to new and exciting discoveries in the field of immunology.
Next TopicArticle Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









