Displacement Reaction DefinitionIntroductionDisplacement reactions are common chemical reactions when another component or ion replaces an element or ion in a compound. A single replacement reaction is another name for this reaction. Displacement reactions are essential for understanding different chemical elements' behavior and other compounds' composition. Displacement reactions occur when a more reactive element or ion replaces a less reactive element or ion in a compound. The driving force behind this reaction is the tendency of some parts to give away electrons or gain electrons to attain a stable electronic configuration. For example, the reaction between copper sulfate and zinc metal can be explained by the difference in reactivity between the two metals. Zinc can substitute copper in the copper sulphate combination because it is more reactive than copper. This reaction results in the formation of zinc sulfate and copper metal. 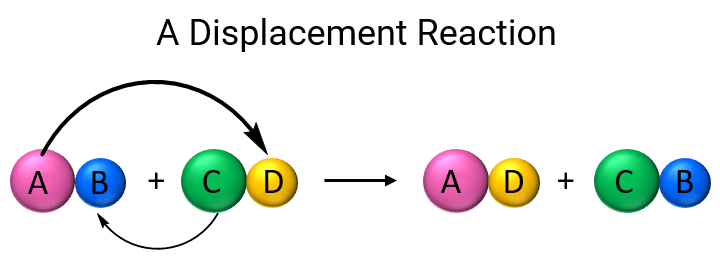
Displacement reactions can occur in aqueous solutions or the solid state. In aqueous solutions, the reaction occurs between two soluble compounds. In the solid state, the reaction occurs between a stable and a solution or between two solids. Displacement reactions are classified into four types: metal displacement, halogen displacement, hydrogen displacement, and displacement of oxygen. Every kind of reaction has unique characteristics; the reactants involved determine the products formed. Displacement reactions have several critical applications in our daily lives. They are used in the production of metals, the extraction of metals from their ores, and the production of batteries. Displacement reactions are also used in the laboratory to test different metals' reactivity and identify unknown substances. What is a Displacement Reaction?A displacement reaction is a chemical reaction where another component or ion replaces one element or ion in a compound. The reaction occurs due to the difference in the reactivity of the two elements or ions. In displacement reactions, the more reactive element or ion replaces the compound's less reactive element or ion. Displacement reactions can occur in different phases, such as in the solid state, liquid state, or gaseous state. For example, when a metal reacts with an aqueous salt solution, the metal may replace the cation in the salt and form a new salt. This type of displacement reaction is commonly known as a metal displacement reaction. An example of a metal displacement reaction is the zinc and copper sulfate solution reaction. In this reaction, zinc metal is added to copper sulfate solution, forming zinc sulfate and copper metal. Halogen displacement reactions are another variety of displacement reactions that take place when one halogen swaps places with another halogen in a compound. For example, when chlorine gas is passed through an aqueous solution of potassium iodide, the chlorine gas replaces the iodine ion in the solution, forming potassium chloride and iodine. Displacement reactions also occur when an element displaces hydrogen from an acid. This type of reaction is known as hydrogen displacement. For example, when zinc is added to hydrochloric acid, the zinc reacts with the acid to form zinc chloride and hydrogen gas. The displacement of oxygen is another type of displacement reaction. A new combination is formed when a compound containing oxygen reacts with a more reactive element. For instance, the reaction between magnesium and oxygen produces magnesium oxide when magnesium is burned in the air. In summary, displacement reactions occur when another component or ion replaces one element or ion in a compound. The type of displacement reaction depends on the reactants involved in the response. Displacement reactions play an important role in many chemical reactions and have numerous applications in industry and everyday life. How does a Displacement Reaction occur?A displacement reaction occurs due to the difference in the reactivity of the two elements or ions. The more reactive element or ion replaces the compound's less reactive element or ion, forming a new combination. Displacement reactions occur when a metal reacts with an aqueous solution of a salt. In this type of reaction, the metal replaces the cation in the salt and forms a new salt. The driving force behind this reaction is the difference in the metal's reactivity and the salt's cation. The more reactive metal displaces, the less reactive cation in the salt. The mechanism of a displacement reaction can be explained by the activity series, a list of metals arranged in order of their reactivity. The metals at the top of the activity series are the most reactive, while those at the bottom are the least reactive. If a metal is placed higher in the activity series than another, it can displace the less reactive metal from its compound. For instance, a displacement reaction occurs when copper metal is added to a solution of silver nitrate, forming silver metal. Because copper is a more reactive metal than silver, it can replace silver in a compound. In halogen displacement reactions, the halogen higher in the activity series will replace the halogen lower in the series from a combination. For example, when chlorine gas is passed through an aqueous solution of potassium iodide, the chlorine gas replaces the iodine ion in the solution, forming potassium chloride and iodine. In summary, displacement reactions occur due to the difference in the element's or ions' reactivity. The more reactive element or ion replaces the compound's less reactive element or ion, forming a new combination. The activity series can predict the outcome of a displacement reaction. Types of Displacement ReactionsDisplacement reactions can be categorized into several types based on the reactants involved. Here are some of the common types of displacement reactions:
In summary, displacement reactions are classified based on the reactants involved in the response. Some of the common types of displacement reactions are metal displacement, halogen displacement, hydrogen displacement, oxygen displacement, carbon displacement, and displacement in double displacement reactions. Factors Affecting Displacement ReactionsDisplacement reactions are influenced by several factors that affect the rate and outcome of the response. Here are some of the critical factors affecting displacement reactions:
In summary, displacement reactions are affected by several factors, including the reactivity series, concentration, temperature, catalysts, surface area, and solvent. Understanding these factors can help predict the rate and outcome of a displacement reaction and optimize reaction conditions for desired results. Applications of Displacement ReactionsDisplacement reactions have numerous applications, from industrial processes to analytical chemistry. Here are some of the critical applications of displacement reactions:
ConclusionDisplacement reactions are fundamental types of chemical reactions that involve the exchange of ions or atoms between two reactants. The reactivity series govern these reactions and can occur in aqueous or non-aqueous solutions or the solid phase. Displacement reactions have numerous applications in various fields, including the extraction of metals, corrosion prevention, chemical synthesis, analytical chemistry, precipitation reactions, and redox reactions. Understanding displacement reactions is critical in developing efficient and effective chemical processes for various applications. Controlling the reaction conditions, such as concentration, temperature, catalysts, and solvent, optimizing the reaction rate and outcome for specific applications is possible. For instance, in the extraction of metals, the use of displacement reactions can minimize the cost of production and reduce the environmental impact of mining activities. In addition, using displacement reactions in corrosion prevention can help extend the lifespan of metal structures and reduce maintenance costs. Precipitation reactions can selectively remove ions from wastewater, contributing to water conservation efforts. On the other hand, Redox reactions have applications in energy storage and production, such as in batteries and fuel cells, which are crucial for developing renewable energy sources. Overall, displacement reactions are a crucial chemistry aspect with broad applications in various fields. Understanding the principles and factors affecting these reactions can enable the development of new applications and the optimization of existing processes. As such, continued research on displacement reactions is necessary to unlock their full potential in addressing current and future societal challenges.
Next TopicDrought Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









