Spectroscopy DefinitionThe field of science deals with spectra of electromagnetic radiation as a fundamental function of its frequency or wavelength calculated by the spectrographic technique or the equipment to get information related to the properties and structure of the matter. Spectral measurement devices are called spectrographs, spectrometers, and spectral analyzers. 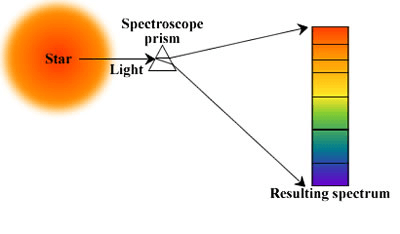
For common understanding, Spectroscopy studies colors as generalized from visible light to all electromagnetic spectrum bands. Spectroscopy has come out as the study of wavelength relying upon the absorption by the gas phase matter of visible light scattered by a prism. It is mainly an electromagnetic spectrum used as the basic tool for the explanation in the branch of physics, astronomy, materials science, and chemistry, and it permits the physical structure, electronic structure of the matter, and composition to be examined at the macro scale, atomic and molecular and over the astronomical distances. Some of the significant applications include medical imaging and biomedical spectroscopy in the field of tissue. The distribution of light through a prism is called to be Optics. Earlier, the study of visible light was referred to as Colour, but later it changed under James Clerk Maxwell and started to be called the electromagnetic spectrum. However, Colour is part of spectroscopy, but this cannot be compared with the Colour of the object and the elements. It is concerned with the reflection and absorption of electromagnetic waves to offer our eyes an idea of Color. But instead, Spectroscopy is concerned with the distribution of light passing through a prism, diffraction grating, or the same equipment producing a typical type of discrete line pattern, referred to as a " Spectrum." It is special to each different form of the element. Those elements that get diffracted when passed through prism-like equipment show an emission or absorption spectrum based on whether the element is heated or cool. Vibrational Spectroscopy: The branch of spectroscopy science deals with studying spectra. Light Scattering spectroscopy is a form of reflectance spectroscopy that decides the tissue structures by investigating elastic scattering. In such a situation, the tissue behaves as a dispersion or diffraction mechanism. Spectroscopic investigations are very crucial to the formation of quantum mechanics. It is due to these studies first atomic model explained the spectra of hydrogen. It included several models like the Schrodinger equation, the Bohr model, and the Matrix mechanics. All these models generated spectral lines of hydrogen and offered a basis for the discrete quantum jump to relate with the discrete hydrogen spectrum. Max Planck involved the Spectroscopy phenomenon in explaining the black body radiation as he was relating the wavelength of the light using a photometer to the temperature of the black body. It is applied in analytical and physical chemistry because molecules and atoms comprise special spectra. The spectra can be applied to quantify, detect and identify the data about the molecules and atoms. It is also applied in remote sensing and astronomy. Research telescopes comprise spectrographs, and calculated spectra are applied to decide the astronomical substance's physical and chemical properties, including black holes, temperature, the density of elements in a star, and velocity. One of the effective uses of spectroscopy is in biochemistry. Forms of Radiative EnergyForms of Spectroscopy are differentiated by the forms of the radiative energy that participated in the interaction. In many uses, the spectrum is determined by calculating the variation in the frequency or intensity of this energy. Forms of radiative energy studies include: Electromagnetic Radiation: It was the first energy source for spectroscopic investigations. Electromagnetic radiation methods are generally categorized by their spectrum wavelength area, consisting of ultraviolet-visible, microwave, near-infrared, gamma, and x-ray spectroscopy. Particles can also be a source of radiative energy due to their de Broglie waves. Both neutron and electron spectroscopy is commonly applied. The kinetic energy decides the wavelength of the particle. Acoustic spectroscopy comprises radiated pressure waves. Dynamic mechanical analysis can be used to give radiating energy just like acoustic waves to solid materials. Behavior of Interaction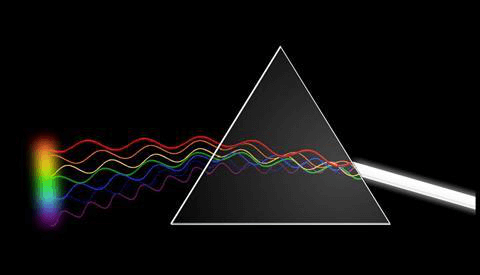
The forms of spectroscopy are differentiated based on the nature of the interaction between the energy and the substance. Some of the interactions include:
Types of MaterialAtomsThe first application of spectroscopy was formed as atomic spectroscopy. Atomic absorption spectroscopy and atomic emission spectroscopy comprise visible and ultraviolet light. These emissions and absorptions are usually referred to as spectral lines because of the electronic transitions of outer shell electrons as they jump and drop from their electron orbit to another. Atoms contain unique x-ray spectra accredited to the excitation of inner shell electrons to the excited states. Atoms of several elements have unique spectra, and because of this, atomic spectroscopy offers the quantitation and identification of a specimen's basic structure. After the inversion of the spectroscope, Gustav Kirchhoff and Robert Bunsen explored the latest elements by observing their emission spectra. The solar spectrum is comprised of Atomic absorption lines, and they are termed Fraunhofer lines after their exploration. A detailed explanation of the hydrogen spectrum was an early triumph of quantum mechanics and described the Lamb shift seen in the formation of quantum electrodynamics. Modern execution of atomic spectroscopy for examining visible and ultraviolet transitions consists of glow discharge, microwave-induced plasma, flame emission spectroscopy, glow discharge spectroscopy, inductively coupled plasma atomic emission spectroscopy, and spark or arc emission spectroscopy. Methods for examining X-ray spectra consist of X-ray Fluorescence and X-ray Spectroscopy. MoleculesThe amalgamation of atoms into molecules leads to distinct types of excited states and, therefore, special ones. ApplicationsSeveral uses of spectroscopy are available in astronomy, medicine, chemistry, and physics. Exploiting characteristics of absorbance and with astronomy emission, spectroscopy can be employed to recognize certain forms of nature. The application of spectroscopy in a different field can be classified as:
Next TopicStory Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









