Element Definition ChemistryChemical elements or elements are such pure compounds that are composed of only one sort of atom. The number of protons in the nuclei of pure elements equals the number of electrons in an atom. Every chemical compound and other material may be found here since they all contain a different element. There are now 118 chemical elements since, to yet, only 118 have been discovered on the planet, some of which are synthetic elements produced via study and some of which are natural elements. 94 elements occur naturally on earth; the remaining elements were either imported from other planets or produced intentionally. The total number of chemical elements reached 118 in 2007. Elements with higher atomic numbers are occasionally generated due to induced nuclear processes, referred to as artificial elements. 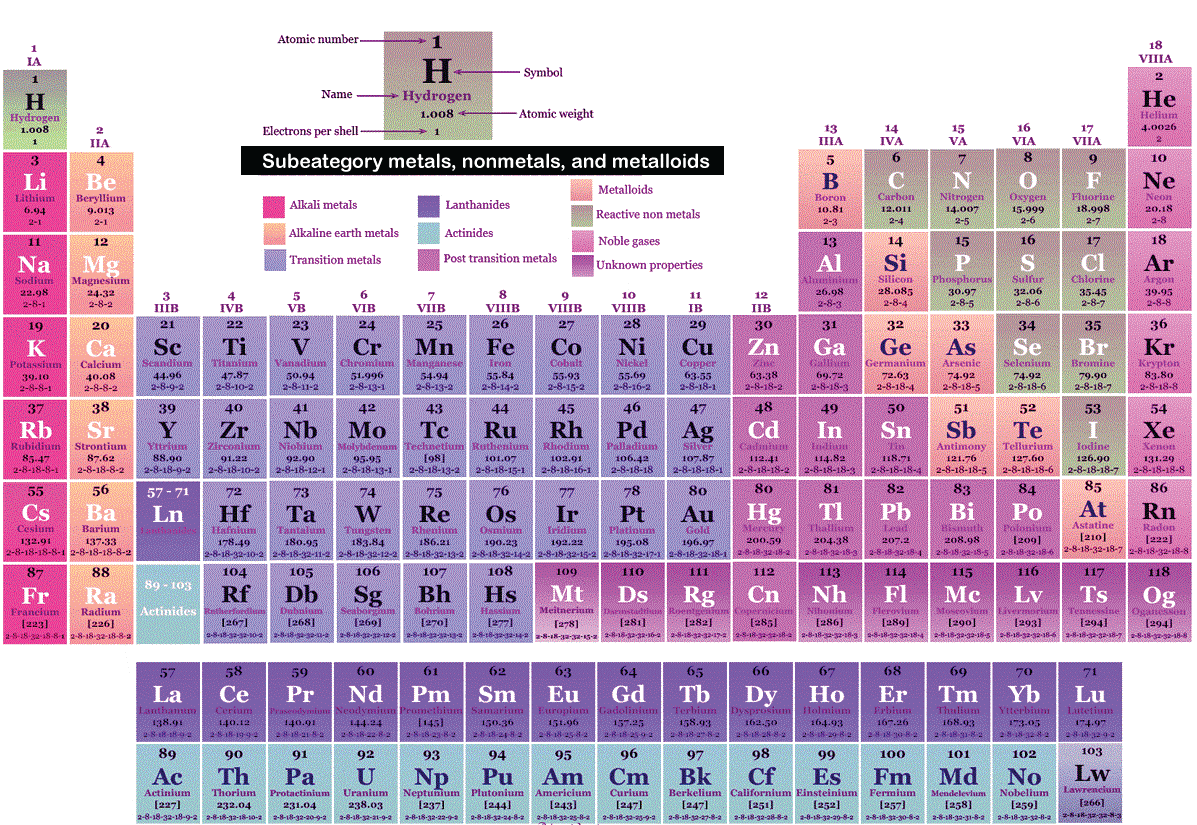
Our scientists have calculated the abundance of each element in our galaxy using spectroscopy. As a result of their calculations, 12 elements were identified that were most abundant in this galaxy, including hydrogen, helium, oxygen, nitrogen, carbon, neon, iron, nickel, potash, Sulphur, magnesium, silicon, and many others. The image above shows the atomic numbers, atomic weights, and symbols of the chemical elements found in nature and those produced artificially. Classification of ElementsThe states of all the elements known so far are different, which are of the following types- MetalMetals are those elements that readily form bonds with other elements. Metals are more helpful than non-metals and more expensive due to their ability to form bonds. Most natural metals may be found in ores made from minerals like calcium, potassium, titanium, iron, and aluminum. Only minerals and ores are used to produce the majority of metals. Metals are substances that readily establish bonds by giving up electrons. Metals and non-metals are the two categories into which elements in chemistry are split. Metals are effective electrical conductors. Typically, metals only exist in their solid form. In other words, metals are those elements that are good heat- and electricity-conductors while also possessing lustrous appearances and high tensile strength. Mankind just recently learned about metal. The Upper Paleolithic era saw the first discovery of copper metal. In the later Vedic era, iron was more identified. However, there are numerous such outliers, such as mercury or mercury, which are termed metals but do not have the characteristics of metals. To claim that mercury is a sort of metal, yet even in its liquid condition, it transforms into jaggery. How many metals are thereAround 118 elements have been identified so far, 91 of which are metals, according to the periodic table. Ferrous metals and non-ferrous metals make up the two main groups into which metals are separated. Both of these kinds of metals have unique characteristics and classifications of their own. The Latin term is where the word ferrous or iron comes from. The term "ferrous metal" refers to a metal with a high concentration of pure iron. if the metal has iron incorporated into it rather than pure iron. More ferrous than non-ferrous metals are utilized. It is manufactured in various methods depending on its content and the amount of molasses. The majority of the commercial and industrial sectors use iron metal extensively. Ferrous metals include cast iron, stainless steel, light weight steel, etc. These metals have extremely high tensile strengths. Non-ferrous elements refer to all pure metals other than iron. Non-ferrous metals include items like aluminum, lead, copper, zinc, and nickel, among others. Non-ferrous metals are those substances, as the name implies, in which iron is not employed. Metal properties and their use in chemicalsMetals are the wealthiest elements. Importantly, metals readily form bonds, which enhances their chemical characteristics. The metals' applications and chemical characteristics are as follows:
Physical Characteristics of MetalMetals have a wide range of chemical and physical characteristics. These characteristics distinguish metals from non-metals. The physical characteristics of metals are as follows:
Uses of MetalsMost decorations are made of metals, including gold, silver, etc. Together with this, ornamental things for the home are also made of metal. Most metals are employed in all work if they are seen. It also creates iron, brass, copper, and other metal utensils. Typically, metal is used to construct a house to create doors, windows, grills, etc. The majority of practically everything manufactured in the world is made of metals. Metal is used in business on a regular basis, in large enterprises, and in factories. If observed, the metal industry has shown to be highly significant and successful in the modern era. Because metal is a good heat conductor, it is also employed in the wire produced for various electrical tasks. In addition, a metal's boiling point is also quite high, along with its melting point. Non-MetalGroups 14 (XIV) through 18 (XVIII) of the periodic table's right-upper quadrant contain them. In addition, the first group's topmost element is a non-metal. In addition to hydrogen, non-metals include zinc, iron, earth, Sulphur, phosphor, halogen, and inert gases. Just 18 elements from the periodic table are often classified as non-metals, whereas more than 80 elements fall within the metals group. Yet, non-metals comprise most of the earth's hydrosphere, atmosphere, and womb. Moreover, non-metals make up the majority of the elements in living things. Nonmetals in the periodic tableIn the top right side of the periodic table are the non-metals. The nonmetal element group typically consists of the following substances, including ideal gases and halogens.
Physical properties of non-metals
Chemical properties of non-metals
Uses of Non-MetalsThe following are some of the main applications for non-metals:
What is the difference between Metals & Non-Metals?
MetalloidsMetalloids are elements that have properties of both metals and nonmetals. They are located between metals and metalloids on Mendeleev's periodic table. Metals may generate chemicals that act as both acids and bases, known as amphoteric substances, and all metals have the property of being semiconductors. Metalloid Examples1. Boron Boron is a chemical sub-metallic element that appears black and glossy and is generated from cosmic rays colliding with an object in the universe. Boron is a Mendeleev periodic table group 13 p-block element with atomic number 5, an atomic mass of 10.80, a melting point of 2200 �C, and a boiling point of 2550 �C. Boron is present in chemical substances on Earth. Boron's compound B2O3 is used to manufacture boric acid, a medication in the glass industry. 2. Silicon Silicon is the most prevalent metalloid element on the planet, with an atomic number of 14, an atomic weight of 28, a melting point of 1414 degrees Celsius, and a boiling point of 3265 degrees Celsius. There are several silicon compounds that are commonly utilized. Silicon is most commonly used in the power, diamond, and electronic industries, which is why the computer center is called as Silicon Valley. 3. Antimony Antimony is a chemical sub-metallic element that occurs naturally in the form of different compounds. It is a part of the p block. It is a glossy and grey metal with an atomic number of 51, an atomic weight of 121.7, a melting point of 630 degrees Celsius, and a boiling point of 1587 degrees Celsius. Sb2S3, an antimony compound, is utilized as an inflammable substance at the tip of matchsticks and in fireworks. Antimony sulfide is also utilised in rubber vulcanization. 4. Germanium Germanium is also known as a chemical brittle submetallic element with an atomic number of 32 and an atomic weight of 72.6. The melting point of germanium is 958 degrees Celsius while the boiling point is 2833 degrees Celsius. Germanium behaves similarly to a semiconductor. As a result, it is employed in producing transistors, optic fibers, photoelectric cells, and solar cells, as a catalyst in polymerization, and various other laboratory operations. 5. Polonium Polonium is a transition metal element with an atomic number of 84 and an atomic weight of 209. Polonium is one such sub-metal with the greatest number of isotopes. 6. Arsenic Arsenic is a chemical sub-metallic element that is predominantly grey and yellow in appearance. Arsenic in grey is brittle and opaque, but arsenic in yellow is translucent. Arsenic has an atomic mass of 74 and an atomic number of 33. Arsenic can be used in various ways since it can generate a range of compounds. Arsenic usage Gallium Arsenide, the most recent substance, is employed in the manufacture of computer chips. 7. Tellurium Tellurium is a brittle, poisonous tin-like metallic element that may be found in the periodic table's P block. It has an atomic weight of 170 and an atomic number of 52. Tellurium is utilised in the production of a wide range of alloys to increase mechanics and in other laboratory processes.
Next TopicEMF Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









