Isomer DefinitionIsomers are molecules that have the same chemical formula but a different arrangement of atoms. There are several types of isomers, including structural isomers, stereoisomers, and enantiomers. 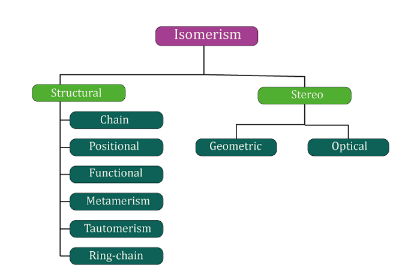
Structural isomersStructural isomers, also known as constitutional isomers, have the same chemical formula but a different arrangement of atoms in the molecule. For example, butane and isobutane are structural isomers because they have the same chemical formula (C4H10) but a different arrangement of atoms. Butane has a linear structure, while isobutane has a branched structure. StereoisomersStereoisomers are isomers that have the same arrangement of atoms but a different spatial orientation of the atoms. There are two types of stereoisomers: geometric isomers and enantiomers.
Optical isomersEnantiomers are a special type of stereoisomers that are mirror images of each other but not superimposable. They are also known as optical isomers. Enantiomers are non-superimposable mirror images of each other, which means that they are identical in every way except for the fact that they are mirror images. For example, the two enantiomers of a molecule of 2-butanol are mirror images of each other, but they are not superimposable. They have opposite configurations at the chiral center and can have different physical and chemical properties, such as melting and boiling points, solubility, and reactivity. Enantiomers also have opposite optical rotation, which means that one enantiomer will rotate plane-polarized light clockwise, while the other enantiomer will rotate it counterclockwise. This property is used to differentiate and purify enantiomers in the field of chiral chemistry. 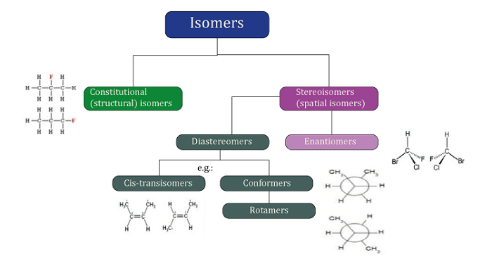
Constitutional isomersStructural isomers, also known as constitutional isomers, have the same chemical formula but a different arrangement of atoms in the molecule. This means that they have the same number of atoms of each element, but they are bonded together in different ways. Structural isomers can be classified into several subcategories, including chain isomers, positional isomers, and functional group isomers.
ConclusionIn conclusion, Isomers are molecules that have the same chemical formula but a different arrangement of atoms. Structural isomers have a different arrangement of atoms in the molecule, stereoisomers have the same arrangement of atoms but a different spatial orientation of the atoms and enantiomers are stereoisomers that are mirror images of each other, but they are not superimposable. Understanding these different types of isomers is important for understanding the properties and reactivity of different compounds.
Next TopicIsotope Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









