Suspension Definition
Suspension is a mechanical mixture where solid particles are dispersed in a fluid. It is a heterogeneous mixture of solid, liquid, or both particles. The solid particles are usually larger than those found in a colloid. The particles are suspended throughout the mixture and are usually not soluble in the mixture. A suspension is a heterogeneous mixture that contains solid particles of different sizes and shapes dispersed in a liquid or gas. The particles can be suspended in the mixture or remain in suspension.
Examples
An example of a suspension is a mixture of clay and water. Clay particles are large enough to be visible to the naked eye and are not soluble in water. The clay particles are suspended in the mixture and can be seen when the mixture is stirred or agitated.
Property of Suspension
The properties of a suspension depend on the size and shape of the suspended particles, the viscosity of the fluid, and the amount of stirring or agitation. The fluid's viscosity affects the rate at which the particles settle out of the suspension. The size and shape of the particles affect the rate of settling. The amount of stirring or agitation affects the time the particles remain suspended.
Types of Suspension
The types of suspensions can be classified as either Colloidal or Non-colloidal. Colloidal suspensions are mixtures of small particles in a fluid, such as milk or blood. The particles are too small to be seen by the naked eye but can be seen under a microscope. The particles are suspended by forces of attraction between them and the fluid. Non-colloidal suspensions are mixtures of large particles in a fluid, such as mud or paint. The particles are large enough to be seen by the naked eye. The particles are held in suspension by the viscosity of the fluid. The minimum size of particles that can be suspended in a suspension depends on the fluid's viscosity and the amount of stirring or agitation. The minimum particle size that can be suspended in a suspension is around 0.1 microns. However, particles smaller than this can be suspended in a colloidal suspension.
Colloidal Suspension
A colloidal suspension is a mixture of two substances, one finely divided and dispersed in the other. The finely divided substance is called a Colloid and is composed of particles ranging from 1 to 1000 nanometers in diameter. Examples of colloidal suspensions include fog, smoke, and milk.
- Dispersion Medium: The dispersion medium is the fluid in which the dispersed phase is suspended. It can be a gas, liquid, or solid, the most common being liquid. The choice of the medium will depend on the application and the desired properties of the mixture. For example, the dispersion medium for painting would be a liquid, such as water or oil.
- Dispersed Phase: This is the material that is dispersed in the medium, and it is usually in the form of particles. The dispersed phase can be solid, liquid, or gas, and the size of the particles will depend on the application. For example, in paint, the dispersed phase would be solid particles of pigment, and in foam, it would be gas bubbles.
- Stabilizing Agent: The stabilizing agent is a substance that helps to stabilize the dispersion. It can be a surfactant, a polymer, or a solid. The type of stabilizing agent used will depend on the application and the desired properties. For example, in paints, surfactants reduce the surface tension between the medium and the particles, and polymers can form a network that helps hold the particles together.
Non-Colloidal Suspension
A non-colloidal suspension is a mixture of two substances, one of which is coarsely divided and dispersed in the other. The coarsely divided substance comprises particles ranging from 1000 nanometers to several millimeters in diameter. Examples of non-colloidal suspensions include mud, sand, and paint.
- Dispersion Medium: The substance in which the non-colloids are dispersed.
- Dispersed Phase: The non-colloids that are suspended in the dispersion medium.
- Coagulating Agent: The coagulating agent is a substance that causes the dispersed phase to form aggregates or clumps. This can be a surfactant, a polymer, or a salt. In paints, surfactants reduce the surface tension between the medium and the particles, and salts can form an insoluble precipitate that helps bind the particles together.
Note: A dispersion is a mixture of two or more components where one component is dispersed in another. In a dispersion, the dispersed component can be a liquid, solid, or gas and is usually suspended in a liquid or gas medium. The size and shape of the particles in the dispersed component depend on the application, and several different stabilizing and coagulating agents can be used to help control the dispersion properties.
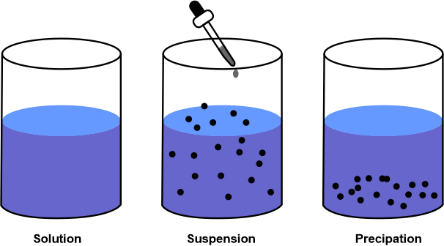
Difference Between Suspension and Solution
- Suspension and solution are two types of mixtures in chemistry. A suspension is a heterogeneous mixture in which the solid particles are distributed throughout a liquid or gas. They are visible to the naked eye and settle after some time if undisturbed. A solution is a homogeneous mixture composed of two or more substances in which a solute is dissolved in a solvent. Solutions are homogeneous, and the particles are not visible to the naked eye.
- The main difference between suspension and solution is that suspension is a heterogeneous mixture in which the particles are visible to the naked eye. In contrast, the solution is a homogeneous mixture in which the particles are not visible to the naked eye. In a suspension, the solid particles do not dissolve in the liquid or gas and remain suspended as a separate entity. On the other hand, in a solution, the solute completely dissolves in the solvent to form a homogeneous mixture.
- Suspension is more visible and easier to observe than a solution, as the solid particles remain suspended in the liquid or gas. The suspension particles can be separated from the mixture by physical methods such as filtration, sedimentation, and decantation. However, the particles of a solution cannot be separated from the mixture by physical methods. They can only be separated by chemical methods such as distillation and crystallization.
- Suspensions are less stable than solutions. The particles of a suspension tend to settle down after some time if undisturbed. The settling time of a suspension depends on the size and weight of the particles. On the other hand, solutions are more stable than suspensions. The particles in a solution remain distributed throughout the mixture even after a long period.
- The viscosity and density of a suspension are greater than that of a solution. This is because the particles of a suspension are larger and heavier than the particles of a solution. The particles of a suspension also scatter light and can be seen as a cloudy or opaque mixture. On the other hand, the particles of a solution do not scatter light, and the mixture appears transparent or clear.
The key difference between suspension and solution is that suspension is a heterogeneous mixture in which the particles are visible to the naked eye. In contrast, the solution is a homogeneous mixture in which the particles are not visible to the naked eye. Additionally, suspensions are less stable than solutions and have higher viscosity and density.
Application of Suspension
- Suspensions can be used in various applications, including filtration, solid-liquid separation, chemical reactions, and the production of pharmaceuticals.
- Suspensions are also used in paints, coatings, and inks.
- Suspension is a common technique used in a variety of scientific applications.
- Suspension can be used to separate mixtures of different substances, such as in chromatography, or to suspend particles in a fluid medium for further analysis.
- Suspension can also be used in medical applications, such as suspending medications in a liquid form for easier ingestion.
- Suspension is also used in various manufacturing processes, such as paint and ceramics.

- Chromatography: Suspension is used to separate mixtures of different materials. In chromatography, a suspension of one material is applied to a solid support, such as a column, and then a solvent is passed through it. The different components of the mixture will be separated as they move through the column.
- Medical Applications: Suspension is often used in medical applications, such as suspending medications in a liquid form for easier ingestion. Suspension can also help separate blood components for various medical tests.
- Manufacturing: Suspension is used in many manufacturing processes, such as for producing paint and ceramics. Suspension is also used to produce certain foods, such as ice cream. Suspension is also used to produce certain chemicals, such as detergents.
In conclusion, suspension is a mechanical mixture where solid particles are dispersed in a fluid. The particles are suspended throughout the mixture and are usually not soluble in the mixture. The properties of a suspension depend on the size and shape of the suspended particles, the viscosity of the fluid, and the amount of stirring or agitation. The minimum particle size that can be suspended in a suspension is around 0.1 microns. Suspensions can be used in various applications, including filtration, solid-liquid separation, chemical reactions, and the production of pharmaceuticals.
|

 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now










