Activation EnergyLet's take a situation where one is waking up a morning with a lot of tasks and fun stuff scheduled ahead, but despite so much exciting and busy days that lie ahead, someone wants to stay more time in bed. Or, it can be said that one needs to muster some extra energy to get themselves out of bed. But once they are up, they are able to coast through the rest of the day, and all thanks to the little hump helped them to get out of the bed. Activation Energy has a similar concept to this hump required for getting out of the bed, but it is not required for any physical process. The activation energy is the hump for a chemical reaction that is required by it to complete the process. But, the actual definition of the activation energy in chemistry or physics is very different from this layman definition. This example is provided here only to clarify the basic concept of activation energy and how it works in a chemical reaction. This article covers the basic details about activation energy, concepts related to it, and what are the uses of activation energy. 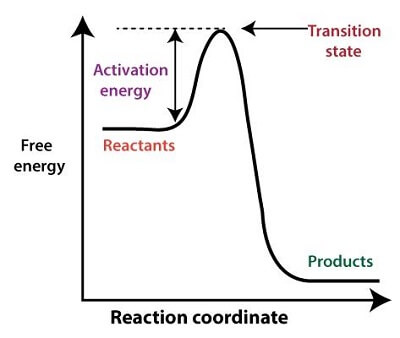
Introduction to Activation EnergyThe activation energy is a term in both physics and chemistry, which is used to define the minimum amount of energy required in a chemical reaction for compounding to the result. The activation energy of a chemical reaction can be considered the magnitude of the potential barrier, which is why it is also known as the energy barrier of a chemical reaction. It means that the activation energy is differentiating minima of the potential energy surface pertaining to the thermodynamic states at the initial and final stages. Svante Arrhenius, the Swedish scientist, was the one who introduced and first used the term activation energy in the year 1889, explaining the concept of the energy barrier for a chemical reaction. The activation energy of a chemical reaction is denoted by the expression 'Ea.' Following are the units in which activation energy of a chemical reaction can be expressed:
Since activation energy is related to the chemical processes or chemical reactions, the use of this term is more dominant in chemistry than in Physics. But that doesn't mean this term or activation energy value has completely no use in the fields of Physics. There are many uses of activation energy in the fields of both physics and chemistry that will be discussed later in this article. Arrhenius equation & Activation EnergyArrhenius provided an equation in which he stated how the activation energy of a molecule or reaction is dependent on multiple factors like temperature, pressure, and others. Arrhenius provided the equation, which is known as the Arrhenius equation, to define the relationship between the rate of a chemical reaction (R) and activation energy (Ea) required by the chemical reaction. Before learning more about this Arrhenius equation and understanding the concepts of it related to the activation energy, let's have a look at the following Arrhenius equation in the simple mathematical variable form: k = Ae-Ea/(RT) The terms used in the Arrhenius equation given above have the following meaning or expansion: (i) k = The 'K' is used to express the reaction rate coefficient of the given chemical reaction (ii) A = The term 'A' used in the equation is used to define a pre-exponential factor used for defining the constant of the reaction (iii) e = The 'e' represents exponential value in the equation which varies according to the activation energy, gas constant, and temperature of the chemical reaction. (iv) Ea: The term 'Ea' used in the above-given equation is used for representing the activation energy of the chemical reaction. (v) R = The term 'R' represents the gas constant for a particular chemical reaction in the equation given above. (vi) T = The term 'T' used in the above-given equation is used for representing the temperature, in Kelvin (K), of the chemical reaction. Looking at the equation given above, it would look like that to find the activation energy of a chemical reaction, all the other expressions should have been given. But this isn't completely true because it is possible to calculate the activation energy of a chemical reaction even without knowing the pre-exponential factor (A) of the equation. This is possible within the validity of the Arrhenius equation as provided above. To calculate the activation energy with knowing the pre-exponential factor (A) of the equation, variations in the temperature of the chemical reaction at various stages should be recorded, and the reaction's rate coefficient (R) should be calculated. Temperature dependence of the activation energy:The activation energy of a chemical reaction varies with the change in the temperature of the reaction. To better understand the dependency of the activation energy of a chemical on the variations in the temperature, first look at the following form of the Arrhenius equation: Ea = RT Log[A/k] According to this form of Arrhenius equation, we can directly conclude the following relation of the activation energy with the temperature of a chemical reaction (When the rate of the reaction is taken as constant): Ea ∝ T This means that the activation energy of a chemical reaction directly varies with the temperature of the reaction. And, if the reaction rate is constant, the activation energy of a reaction can be calculated with the variations in the temperature. This is because the gas constant of any chemical reaction is a constant value, and it doesn't vary with the changes in the other factors of the reaction. At the same time, the activation energy is also indirectly proportional to the rate coefficient of a reaction when the temperature of the reaction remains constant. Negative Activation EnergyIn some cases, it is even seen that the activation energy of the chemical reaction decreases with an increase in the temperature. This usually happens when the temperature of the chemical reaction increases rapidly. Coming back to the Arrhenius equation, to fit the rate constant in the equation with the increasing exponential relationship, the activation energy of the reaction comes out in a negative value as a result. Typical barrierless reactions, the type of elementary reaction that usually exhibits negative activation energy, follow the capture of the molecules in a potential well according to which reaction proceeding relies on. The rapid increase in the temperature decreases the probability of the colliding molecules (because of glancing collision) capturing one another, which is expressed as the reaction cross-section. The chances of seeing this phenomenon during a chemical reaction decrease with an increase in temperature. Note: The type of molecule collisions that do not lead to reaction because the higher momentum of the reaction carries the colliding particles out of the potential well is known as the glancing collision.Uses of Activation EnergyThe activation energy plays a crucial role in calculating factors that will be essential to know before starting a chemical reaction or any other physical process. Although the activation energy term is used in many places, it is crucial to determine many important factors and derive crucial data regarding many physical and chemical processes. Following are some areas or fields where the calculation of activation energy is required or is used as a factor for deriving important information regarding physical processes: (i) Calculation of rate of reaction (ii) Temperature required to maintain equilibrium in a chemical reaction (iii) Used in thermodynamics (iv) Also applies to nuclear energy (v) To find the nature of a chemical reaction and in various other physical and chemical phenomena.
Next TopicLattice Energy
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









