Unit of heatThe S.I unit of heat is Joule. One Joule is defined as the amount of work done on a body when a force of 1 Newton displaces a body at a distance of 1m in the direction of the force. 1 Joule = Nm 1 N = 1kg m/s2 1 Joule = kg m/s2 x m 1 Joule = kg m2/s2 Other forms of JouleLet's discuss other units in the form of Joule. 1 Joule = Pa. m3 It is because 1 Joule = Nm 1 N = 1 Pa.m2 1 Joule = Pa.m2. m = Pa. m3 Where, P is Pascal N is Newton m is meter 1 Joule = Ws 1 Watt = Joules/second 1 Joule = Watt x second Thus, 1 Joule = W.s Where, W is the Watt s is the seconds 1 Joule = C.V 1 Joule = W.s = Watt. Seconds Watt is the unit of Power Power = Voltage x Current Unit of current is Amperes and unit of voltage is volts 1 A = Q/t = charge in coulomb / time in seconds P = V x Q/t Converting the above equation in terms of their units, we get: 1 Watt = Volts x Coulomb/seconds We know that: 1 Watt = Joules/second Joules/ second = Volts x Coulomb/seconds J/ s = V x C / s J = VC Or J = CV Hence, 1 joules = C.V Other units of heatThe other units of heat are given by:
Let's discuss the above two units of heat in detail. CaloriesA calorie is also known as the unit of energy, and it refers to the amount of energy our body burns or the amount of energy it gets from eating or drinking. The calories can be expressed in gram calories or kilocalories. 1 calorie = 4.184 Joules 1 kcal (kilocalories) = 4184 Joules Or 1 joule = 0.239006 calories 1 joule = 0.000239006 kcal (kilocalories) BTUBTU refers to the British Thermal Unit. It is defined as the amount of heat required at the constant pressure to raise the temperature of one pound of the liquid (water) by one degree Fahrenheit. It generally measures the heat content of the fluids, such as fuels. 1 Btu = 1055.06 Joules 1 Btu = 1.05506 kJ (Kilo Joules) Or 1 Joule = 0.000947817 Btu 1 kJ (Kilo Joules) = 0.947817 Btu Multiples of JoulesThe unit of heat, i.e., Joule, is also expressed in the multiples, which is listed as follows:
What is heat?Heat is defined as energy, which is transferred from one system to another at different temperatures. The heat generally flows from the high-temperature system to the low-temperature system. Heat formulaThe heat formula is given by: 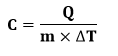
Where, C is the specific heat of the body M is the mass of the body Q is the heat T specifies the temperature or the change in temperature Numerical ExamplesLet's discuss some Numerical Examples on heat. Example 1: Find the heat required to raise the temperature of the water of mass 0.7 g from 30 degrees Celsius to 90 degrees Celsius? Solution: The parameters given are: Change in temperature = 90 - 30 = 60 degrees Celsius. The specific heat of water is 4186 J/g degrees Celsius Mass of water be 0.7 g. The heat formula is given by: 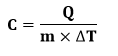
Heat (Q) = 4186 x 60 x 0.7 = 175812 Joules Heat = 175.812 Kilo Joules Thus, the heat required to raise the temperature of the water from 30 degrees Celsius to 90 degree Celsius is 175.812 Kilo Joules. Example 2: Find the specific heat of water mass f 10 kg if the temperature changes from 130 degrees Celsius to 100 degrees Celsius. The heat required is 120 kcal. Solution: The formula to find the specific heat is given by: 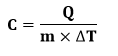
The mass of the water is 10 kg = 10000 g. Change in temperature is 130 - 100 = 30 degrees Celsius. Heat (Q) = 120 calories So, we will first convert the calories into the Joules. We know that 1 calorie = 4.184 Joules So, 120 kilo calories = 120 x 4.184 = 502080 Joules Specific heat (C) = 502080J/10000 g x 30 degrees Celsius = 1.6736 J/g degree Celsius Thus, the specific heat of water is 1.6736 J/g degrees Celsius.
Next TopicHeat Engine
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









