Difference between Density and VolumeIntroduction:When we examine the properties of matter, two fundamental concepts often come into play: density and volume. These properties help us comprehend how substances occupy space, how tightly their particles are packed, and their mass distribution within that space. While they are related, density and volume represent distinct aspects of matter. Density: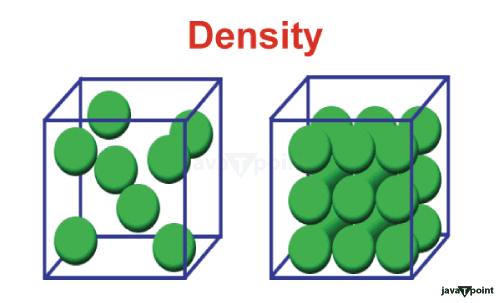
Definition: Density is a fundamental property that quantifies how much mass is contained within a given volume of a substance. It essentially tells us how tightly the particles, be they atoms, molecules, or other constituents, are packed within a material. Units: Density is typically expressed in units like kilograms per cubic meter (kg/m�) in the International System of Units (SI) or grams per cubic centimeter (g/cm�) in the metric system. Formula: The formula for density is straightforward: ? = m/V, where ? represents density, m stands for mass, and V denotes volume. Significance: Density is a crucial property in materials science, physics, and engineering. It allows us to identify substances, determine their purity, and predict their behavior under various conditions. For example, it explains why oil floats on water (oil has a lower density than water), and it's a key factor in understanding the behavior of fluids, especially in the context of buoyancy. Applications: Density finds applications in a wide range of fields, from chemistry to engineering. It's used in material science to classify materials, in geology to identify minerals, and in the construction industry to assess the strength of materials. Volume:Definition: Volume represents the amount of space occupied by an object or substance. It is a measure of the three-dimensional extent or size of an object. Units: Volume is typically measured in cubic units such as cubic meters (m�) in SI units, cubic centimeters (cm³) in the metric system, or in liters (L) and milliliters (mL) for practical purposes. Formula: Calculating volume depends on the shape of the object. For regularly shaped objects like cubes or spheres, there are specific formulas. For irregular shapes, experimental techniques like water displacement are used to measure volume accurately. Formula: Calculating volume depends on the shape of the object. For regularly shaped objects like cubes or spheres, there are specific formulas. For irregular shapes, experimental techniques like water displacement are used to measure volume accurately. Applications: Volume is widely used in various fields, including physics, architecture, cooking, and more. In chemistry, precise measurements of volume are essential for mixing chemicals in the correct proportions. Difference between Density and Volume:Key differences between density and volume: Nature:
Units:
Calculation:
Use:
In summary, density, and volume are distinct properties with different meanings and units, but they are related when considering the mass of a substance within a given space. Understanding these concepts is essential in various scientific and engineering contexts, including physics, chemistry, and materials science. Similarities between Density and Volume:
Despite these similarities, it's crucial to recognize that density and volume represent different aspects of matter. Density quantifies how tightly particles are packed within a substance, while volume measures the space occupied by a material. Understanding these distinctions is essential for a comprehensive grasp of the physical properties of substances. Conclusion:In conclusion, density and volume are fundamental properties that help us make sense of the physical world. They are essential concepts in science and engineering, enabling us to identify materials, predict behavior, and solve practical problems. Understanding these properties is not only vital in academic and scientific pursuits but also in our everyday interactions with the physical world. |
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









