Surface EnergySurface energy, also known as interfacial energy, is a fundamental property of materials that describes the energy required to form or maintain a surface or interface between two phases of matter. It is a measure of the strength of the attractive forces between the atoms or molecules at the surface of a material and the surrounding medium, whether it is a gas, liquid or solid. 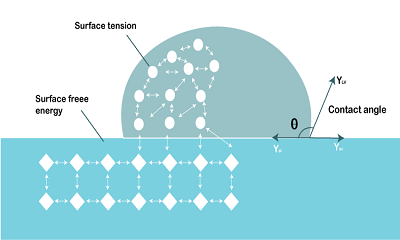
The concept of surface energy is relevant in a wide range of fields, including materials science, chemistry, physics, and engineering. It is crucial in understanding the behavior of materials in various applications, such as adhesion, wetting, and surface tension, as well as in the study of biological systems, such as cell membranes and proteins. Surface energy is defined as the excess energy per unit area of the surface or interface between two phases of matter. In other words, it is the energy required to increase the surface area of a material by a certain amount. This definition implies that the surface energy of a material is always greater than the bulk energy, since the creation of a new surface introduces additional interactions between the atoms or molecules at the surface and the surrounding medium. Unit of Surface EnergyThe unit of surface energy is typically expressed in terms of energy per unit area, such as joules per square meter (J/m2) or ergs per square centimeter (erg/cm2). Both units represent the amount of energy required to create or maintain a surface or interface between two phases of matter. Joules per square meter is the SI unit for surface energy and is defined as the amount of energy required to create a surface with an area of one square meter. This unit is commonly used in scientific and engineering applications. For example, the surface energy of a material can be measured in J/m2 using techniques such as contact angle measurements or surface tension measurements. Ergs per square centimeter is another unit commonly used to express surface energy. It is a smaller unit than J/m2 and is often used in physics and chemistry. One erg is equivalent to 10-7 joules, so the surface energy expressed in ergs per square centimeter is typically a smaller value than the same energy expressed in J/m2. In some cases, other units may also be used to express surface energy, such as electron volts per square angstrom (eV/Å2) or calories per square centimeter (cal/cm2). These units are less common and are typically used in specialized fields of study. Regardless of the unit used, the surface energy of a material is always expressed in terms of energy per unit area. This is because the creation or maintenance of a surface or interface involves the transfer of energy to or from the material, and the amount of energy involved is proportional to the area of the surface or interface. Therefore, the unit of surface energy is an essential component of measuring and comparing the surface energies of different materials. Factors affecting Surface EnergySurface energy is a fundamental property of materials that is influenced by several factors. Understanding these factors is crucial in controlling the behavior of materials in various applications, such as adhesion, wetting, and surface tension.
Calculation of Surface EnergySurface energy is a physical quantity that describes the amount of energy required to create or maintain a surface. It is also referred to as interfacial energy, as it applies to the interface between two phases or materials. Surface energy is an important concept in materials science and engineering, as it plays a critical role in determining the properties and behavior of materials. The calculation of surface energy involves determining the energy required to create or maintain a surface. This can be done using several different methods, including theoretical calculations, experimental measurements, and empirical models. Theoretical calculations of surface energy are based on fundamental principles of physics and chemistry. One of the most widely used theoretical models for calculating surface energy is the Young equation, which relates the contact angle of a liquid droplet on a solid surface to the surface energy of the solid. The Young equation is given by: cos(theta) = (gamma_sv - gamma_sl) / gamma_lv where theta is the contact angle, gamma_sv is the surface energy of the solid-vapor interface, gamma_sl is the surface energy of the solid-liquid interface, and gamma_lv is the surface energy of the liquid-vapor interface. By measuring the contact angle of a liquid droplet on a solid surface and knowing the surface energies of the liquid and solid, the surface energy of the solid can be calculated using the Young equation. Experimental measurements of surface energy can be done using a variety of techniques, including contact angle measurements, surface tension measurements, and atomic force microscopy. Contact angle measurements involve measuring the angle formed between a liquid droplet and a solid surface. The contact angle is related to the surface energy of the solid using the Young equation. Surface tension measurements involve measuring the force required to pull a liquid surface apart, and the surface energy is calculated from the surface tension using the equation: gamma = F / (2L) where gamma is the surface energy, F is the force required to pull the surface apart, and L is the length of the surface being pulled. Atomic force microscopy can be used to directly measure the force required to break molecular bonds at the surface of a material, providing a direct measurement of surface energy. Empirical models for surface energy are based on experimental data and provide a way to estimate surface energy without requiring detailed measurements or theoretical calculations. One of the most widely used empirical models is the Owens-Wendt model, which is based on the idea that the surface energy of a material is related to its polar and dispersive components. The Owens-Wendt model is given by: gamma = gamma_d + gamma_p where gamma_d is the dispersive component of the surface energy, which is related to the nonpolar interactions between molecules, and gamma_p is the polar component of the surface energy, which is related to the polar interactions between molecules. The polar and dispersive components can be estimated from the surface tension of various liquids, and the surface energy of a material can be calculated using the Owens-Wendt equation.
Next TopicThreshold Energy
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









