Free EnergyFree energy is a thermodynamic parameter that gauges how much energy is accessible in a system to carry out meaningful work. Thermodynamics uses this idea to forecast how physical and chemical systems will behave. Free energy, expressed simply, is the energy that can operate in a system without any external energy input. 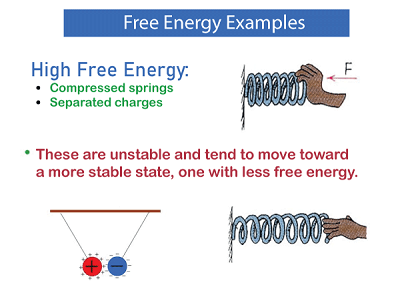
An indicator of how much energy is available in a system to perform useful work is the thermodynamic concept known as free energy. In order to predict how physical and chemical systems would behave, thermodynamics exploits this concept. The energy that can function in a system without the addition of outside energy is known as free energy. Free energy is expressed in units of energy, such as joules or calories. It is typically denoted by the symbol G and is defined as the difference between the enthalpy (H) and the product of the absolute temperature (T) and the entropy (S) of a system, multiplied by a constant factor of -1: G = H - TS where G is the free energy, H is the enthalpy, T is the absolute temperature, and S is the entropy. The enthalpy of a system is a measure of the system's total energy, including the molecules' potential energy and the energy associated with the bonds between the molecules. The entropy of a system is a measure of the degree of disorder in the system. When a system undergoes a change, the change in enthalpy and entropy determine the change in free energy. 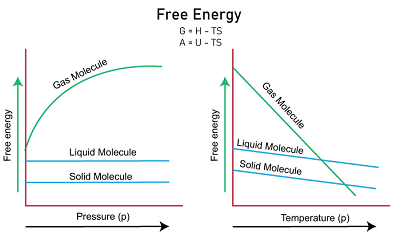
A system's free energy can be used to predict whether a chemical reaction will occur spontaneously. The reaction will happen spontaneously and release energy that can be used for work if the free energy of the products is lower than the free energy of the reactants. A source of energy will be needed to drive the reaction if the free energy of the products is larger than the free energy of the reactants. In this case, the reaction will not take place spontaneously. Free energy is a useful concept in many areas of science and engineering, including chemistry, physics, biology, and materials science. It is used to predict the behavior of chemical reactions, the stability of materials, and the efficiency of energy conversion processes. Types of Free EnergyThere are several different types of free energy that are commonly used in science and engineering. These include Gibbs free energy, Helmholtz free energy, and internal energy. Gibbs free energy (G):The most prevalent kind of free energy in chemistry and thermodynamics is Gibbs free energy, indicated by the letter G. It is described as the quantity of energy present in a system at constant temperature and pressure that may do productive work. Gibbs free energy is used to determine whether a chemical reaction will occur spontaneously by taking into account the enthalpy and entropy of a system. Gibbs free energy's formula is G = H - TS, where G stands for Gibbs free energy, H for enthalpy, T for absolute temperature, and S for entropy. If the Gibbs free energy of a reaction is negative, the reaction will occur spontaneously in the forward direction, releasing energy that can be used to do work. If the Gibbs free energy of the reaction is positive, the reaction will not occur spontaneously in the forward direction, and an input of energy will be required to drive the reaction. Helmholtz free energy (A):A represents Helmholtz free energy, a type of free energy that is frequently applied in physics and engineering. It is described as the quantity of energy present in a system at constant volume and temperature that may do useful work. A system's internal energy and entropy are taken into consideration by the Helmholtz free energy, which is used to forecast how systems will behave when they are in equilibrium. The equation for the Helmholtz free energy is A = U - TS, where A is the Helmholtz free energy, U is the internal energy, T is the absolute temperature, and S is the entropy. Helmholtz free energy is used to predict the behavior of systems in equilibrium, such as mechanical systems and electrical circuits. In a system at equilibrium, the Helmholtz free energy is at a minimum, and any change in the system will increase the Helmholtz free energy. Internal energy (U):Internal energy, denoted by the symbol U, is a type of free energy used to describe a system's total energy. It includes the kinetic energy and potential energy of the molecules in the system. Internal energy is related to the enthalpy and entropy of a system through the equation: U = H - PV where U is the internal energy, H is the enthalpy, P is the pressure, and V is the volume. Internal energy is used to predict the behavior of systems under different conditions, such as temperature and pressure changes. When a system undergoes a change, the internal energy of the system changes, and this change can be used to predict the behavior of the system. Free energy of formation (ΔGf):The free energy of formation, denoted by the symbol ΔGf, is a type of free energy that is used to describe the energy required to form a compound from its constituent elements. The free energy of formation is defined as the change in free energy that occurs when one mole of a compound is formed from its constituent elements in their standard states. The equation for the free energy of formation is: ΔGf = Gf(products) - Gf(reactants) where ΔGf is the free energy of formation, Gf(products) is the free energy of the products, and Gf(reactants) is the free energy of the reactants. The free energy of formation is used to predict the stability of compounds and the energy required to form them. It is also used to calculate the energy released or absorbed during a chemical reaction. Applications of Free EnergyThe concept of free energy has a wide range of applications in various fields, including physics, chemistry, engineering, and biology. Here are some of the key applications of free energy: Chemical reactions and thermodynamics:Free energy plays a critical role in predicting whether a chemical reaction will occur spontaneously or not. Gibb's free energy, in particular, is used to determine the feasibility of a reaction at a given temperature and pressure. If the Gibbs free energy is negative, the reaction will occur spontaneously, while if it is positive, the reaction will not occur spontaneously. The concept of free energy is also used to study the thermodynamics of materials and their properties, such as melting point, boiling point, and phase transitions. Renewable energy:Free energy is a key concept in the development and use of renewable energy sources such as wind, solar, and hydroelectric power. The energy available from these sources is essentially free, as it is derived from natural processes and does not require burning fossil fuels or other non-renewable resources. The availability of free energy from renewable sources has spurred the development of new technologies, such as solar cells, wind turbines, and hydroelectric generators, that can convert this energy into usable forms. Biological systems:Free energy plays a critical role in biological systems, from the molecular level to the organismal level. The concept of free energy is used to study the behavior of enzymes, proteins, and other biomolecules, as well as the metabolic pathways that occur in living cells. In biological systems, free energy is often used to drive chemical reactions, such as the conversion of glucose into ATP, which is the primary energy currency of cells. Free energy is also used to study the thermodynamics of biological processes, such as protein folding, DNA replication, and gene expression. Materials science:Free energy is a critical concept in materials science, particularly in the study of phase transitions and the behavior of materials at high temperatures and pressures. The free energy of a material can be used to predict its stability, its melting point, and its response to changes in temperature and pressure. Free energy is also used to study the behavior of materials under different conditions, such as the behavior of metals at high temperatures or the behavior of ceramics in extreme environments. Engineering:Free energy is a key concept in engineering, particularly in the design and optimization of energy systems. Engineers use free energy concepts to design and optimize energy-efficient systems, such as heating and cooling systems, engines, and power plants. Free energy is also used in the development of renewable energy technologies, such as wind turbines, solar cells, and hydroelectric generators. Engineers use free energy concepts to optimize the efficiency of these systems and to design new technologies that can convert free energy into usable forms. Information theory:Free energy is a concept that has been used in information theory to describe the minimum amount of work required to process information. In this context, free energy is used to describe the amount of work required to extract useful information from a signal, such as a radio signal or a digital image. Free energy is also used in the study of artificial intelligence and machine learning, where it is used to describe the amount of work required to train a neural network or other machine learning algorithm.
Next TopicStrain Energy
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









