Lattice EnergyThe lattice energy, a topic of chemistry and thermodynamics, is a term used to define the ion strength of the ionic compounds, including both alkali and alkaline type compounds. This term is also used in thermodynamics to find out how a compound or chemical reaction can reach its equilibrium state without affecting the Stereochemistry of the compound. Therefore, calculating the lattice energy of ionic compounds becomes important, and it should be provided while dealing with many chemical compounds in a laboratory. 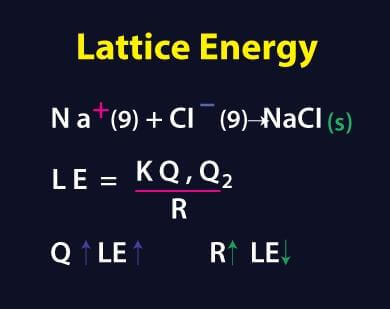
Lattice energy is not a complex or big topic, but it is important to be covered while studying ionic compounds or thermodynamics in chemistry. Therefore, studying this topic becomes a must while learning the chemistry of compounds and reactions, and it should also not be left uncovered while working on ionic compounds in the laboratory. This article talks about the basic concepts of lattice energy, attributes of lattice energy, and factors affecting lattice energy. Note: Stereochemistry: It is a sub-discipline of the chemistry branch which deals and talks about the formation of atoms & molecules and their spatial arrangements under various environments & reactions.Introduction to Lattice EnergyAccording to the classical definition given in chemistry, "Lattice energy is the total amount of energy required for breaking or converting one mole of aqueous ionic compounds into one mole of ions." That's how lattice energy is used as a quantity that can tell us about the strength of bonds in the ionic compounds. If an ionic compound requires greater lattice energy to convert compound into gaseous ions, it means that the strength of ionic bonds in these compounds is very much stronger. This also holds true in the opposite cases; lower the amount of lattice energy required, weaker the bond strength of ionic compounds. The lattice energy of an ionic compound is also helpful in providing some basic insights into the several properties of the compound, such as solubility, visibility, and many others. One thing that should be noted here is that the lattice energy of any compound cannot be calculated directly. It means that there is no formula for calculating the lattice energy of a compound based on given values, and it can only be derived after multiple laboratory calculations and experiments. Usually, the lattice energy of any ionic or chemical compound is deduced from the 'Born-Haber Cycle', and value is provided in the result. Alternate Definition of Lattice Energy: There is one alternate definition of lattice energy of a compound which states, "The total amount of energy required by one mole of ionic compounds present in the gaseous state in order to separate the ions of the compound in gaseous form, as a result, is known as the lattice energy." Lattice Energy and Lattice EnthalpySometimes, many chemistry students get confused between these two terms (lattice energy and lattice enthalpy), but these two are very different and are used to represent two different values & measurements. These two are similar values but are not exactly the same, and that's why before proceeding further, it is important first to understand the term lattice enthalpy. The lattice enthalpy is a term that comes under the lattice energy topic, and it is used to describe the strength of forces between ions of an ionic compound. The lattice enthalpy can be used to describe the strength of forces between ions of the same atoms as well as ions of different atoms. At the same time, lattice energy is used to describe the bond strength of ionic compounds and the force of attraction between ions of different atoms. Value of Lattice EnergyBreaking bonds in an ionic compound to separate its ions into the gaseous state is done through an endothermic process (A chemical reaction or process where energy is consumed during the process). Therefore, the lattice energy value of an ionic compound is always positive and greater than 0. For example, Let's have a look at the following chemical reaction: NaCl (s or aq) => Na+ (g) + Cl- (g) In this reaction, a simple ionic compound (Sodium chloride salt) is separated into its ions in gaseous forms (Sodium and Chlorine), but to proceed with or complete this reaction, some amount of energy is required, which is known as lattice energy of the ionic compound. The numerical value of lattice energy for separating the sodium chloride compound into sodium and chlorine ions is 786 kilojoules (KJ). This lattice energy value is calculated through laboratory experiments and using the values derived through the 'Born-Haber Cycle.' Representative Lattice energy values:Like the lattice energy of sodium chloride (NaCl) as discussed above, there are also many other common ionic compounds whose lattice energy is given. And the representative lattice energy values of these common ionic compounds are used in calculating many other properties of them. Therefore, representative lattice energy values of many common ionic compounds are given below with a brief comment on their lattice energy value and lattice structure: (i) NaCl (786 KJ/mol): This ionic compound's lattice energy is slightly moderate because of the higher difference in the charge & radius values of cations and anions in the compound. (ii) LiF (1030 kJ/mol): The structure type of the Lithium Fluoride ionic compound is NaCl (it means that this compound has a similar structure pattern as of NaCl). This compound's lattice energy is higher because of the lower difference in the charge values of cations and anions in the compound. (iii) NaI (704 KJ/mol): The lattice structure of this ionic compound is also the NaCl type, but this compound has weaker ionic bonds, and that's why the lattice energy value of this compound is very low. (iv) NaBr (747 kJ/mol): The NaBr also has the lattice structure of NaCl type, but the lattice energy of this compound is much lower because of the weaker forces present between the ionic bonds of this compound. The charge value difference of anions and cations in this compound is much higher than in NaCl. (v) CaO (3414 KJ/mol): The CaO is double charged ionic compound in which ions break into Ca2+ and O2-. The CaO also has the NaCl-type lattice structure, but this ionic compound has much higher lattice energy because of the higher lattice enthalpy of the ions. The CaO is insoluble in water, making it even harder to break the ionic bond present in this compound. (vi) MgO (3795 KJ/mol): Despite having the same lattice structure as CaO and NaCl, the lattice energy of MgO is very high because of the higher lattice enthalpy of the ions and lower difference charge value of the elements present in this compound. (vii) SrO (3217 KJ/mol): The SrO also has the NaCl type lattice structure, but this ionic compound has much higher lattice energy because of the higher lattice enthalpy of the ions. (viii) CsCl (657 kJ/mol): This ionic compound's lattice energy is slightly lower because of the higher difference in the charge & radius values of cations and anions in the compound. This ionic compound has its own type of lattice structure, which is also seen in many other ionic compounds. (ix) CsI (600 kJ/mol): The lattice structure of this ionic compound is also the CsCl type, but this compound has weaker ionic bonds, and that's why the lattice energy value of this compound is very low. (x) CsBr (632 kJ/mol): Despite having the same lattice structure as CsCl, the lattice energy of CsCl is low because of the lower lattice enthalpy of the ions and higher difference charge value of the elements present in this compound. Factors affecting Lattice EnergyThe lattice energy is mainly affected because of the ionic bonds and ions present in the compound, but these factors do not directly affect the lattice energy of an ionic compound. The magnitude of charge and distance associated with these factors affects the lattice energy. The description of these two factors that affect the lattice energy of a compound is given below: (1) Distance between the two ions: The total distance in the two ions of an ionic compound plays a crucial role in determining the lattice energy of the compound. More the distance between two ions, the weaker the ionic bond present between them that requires less lattice energy to break this bond and free the ions of the compound. Opposite to this, less the distance, more lattice energy is required to break the ionic bond between two ions. (2) Magnitude of the charge on constituent ions: The charge value of the ions present in an ionic compound also plays a crucial role in determining the lattice energy of the compound. Due to the electrostatic force present in the ionic compounds, the ions of an ionic compound are also attracted to each other with great magnitude. The strength of this attraction is directly connected to the magnitude of the charge of the ions that forms the ionic bond. The higher the magnitude of charge in the ions, the more strongly the ions will be attracted towards each other, making more lattice energy required to break the ionic bond.
Next TopicWind Energy
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










