What is the Charge of a Proton in Coulombs?Proton charge in CoulombsA proton's charge is equivalent to and in opposition to an electron's charge. A proton has a charge of +1.610-19 coulomb (C), while an electron has a charge of -1.610-19 coulomb (C). 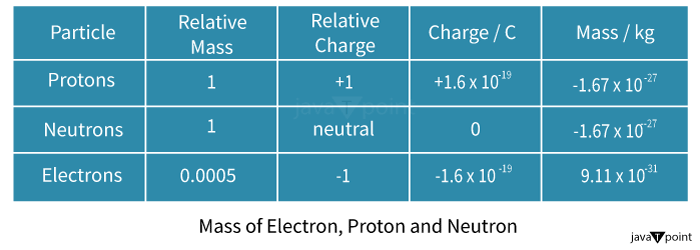
Charge in Coulomb's Law
Proton MeaningA proton is a stable subatomic particle with the symbols p, H+, or 1H+ and an electric charge of +1 e. (elementary charge). Its mass is much smaller than a neutron and 1,836 times bigger compared to that of an electron (the proton-electron mass ratio). Protons and neutrons, each of which has a mass of about one atomic mass unit, are together referred to as "nucleons." (particles present in atomic nuclei). A variety of protons are present in the nucleus of each atom. They provide the main electrical attraction and holding power for the atomic electrons. An element's atomic number, or the quantity of protons in the centre of it (denoted by the sign Z), is what distinguishes it from other elements. Since every element has a specific number of protons, every element also has a different atomic number, which establishes the number of atomic electrons and, therefore, the element's chemical properties. The Greek term for "first" is a proton, and Ernest Rutherford gave the hydrogen nucleus this name in 1920. Rutherford had previously established that atomic collisions might separate the nitrogen nuclei from the hydrogen nuclei, which is believed to be the least dense nucleus. Since they are the building blocks of nitrogen & all other heavier atomic nuclei, protons are a candidate for the title of fundamental or elementary particle. These electrons, according to Rutherford, should revolve around a positive nucleus. In further experiments, he discovered that the nucleus contains a proton, a tiny positively charged particle. A third subatomic component, known as a neutron, also exists. Properties of Proton
History of Proton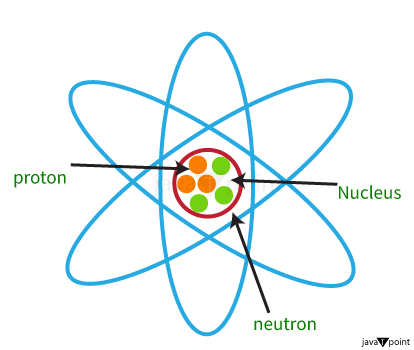
Over a lengthy period of time, the idea that a particle similar to hydrogen could make up additional atoms came into being. William Prout suggested the idea that all molecules are made up of atoms of hydrogen (which he referred to as "protyles") as early as 1815. Still, this idea was later discredited when more precise measurements of atomic weights were made. Canal rays, commonly referred to as anode rays, were first identified by Eugen Goldstein in 1886, and he demonstrated that they contained positively charged atoms (ions) formed from gases. Contrary to J. J. Thomson's discovery of negative electrons, these particles could not be distinguished by a single particle because they had different charge-to-mass ratios (q/m) from those from various gases. The particle in ionized gases with the largest charge-to-mass ratio, according to Wilhelm Wien's classification in 1898, is the hydrogen ion. After Ernest Rutherford discovered the atomic nucleus in 1911, Antonius van den Broek argued that each element's position in the periodic table (atomic number) corresponds to its nuclear charge. Henry Moseley conducted an experiment in 1913 and used X-ray spectra to demonstrate this. Rutherford demonstrated in 1917 (in tests documented in 1919 and 1925) that the nucleus of hydrogen is present in different nuclei, a finding that is typically referred to as the discovery of protons. Rutherford found that his scintillation detectors produced the signs of typical hydrogen nuclei when alpha particles were fired into the air (mainly nitrogen), which is when these investigations got their start. After conducting experiments, Rutherford connected the chain reaction to the nitrogen within the atmosphere and discovered that the reaction was amplified when alpha particles were added to pure nitrogen gas. Rutherford made the erroneous assumption in 1919 that the alpha particle basically stripped a proton from nitrogen, converting it to carbon. Rutherford discovered that the alpha particles were absorbed in 1925 after viewing Blackett's cloud chamber photos. After the alpha particle is captured, its nucleus of hydrogen is released, causing heavy oxygen to be produced rather than carbon, increasing the nucleus' atomic number Z rather than decreasing it. 14N +α= 17O + p was the first nuclear reaction to be observed. At first, Rutherford mistook the contemporary "p" in the formula for a hydrogen ion, or H+. Depending on your point of view, the proton's "discovery" can be attributed to either 1919, when it was observed experimentally as deriving from a source other than hydrogen, or 1920, when it was identified and postulated as an elementary particle. Prout's theory that hydrogen served as the fundamental unit of all other elements impacted Rutherford, who was aware of hydrogen's simplicity and lightness. Rutherford gave the hydrogen nucleus H+ a particular name because he believed that hydrogen, the element with the lowest mass, had only one of these particles. However, it was later discovered that the hydrogen nucleus can be discovered in other nuclei as a fundamental particle. The neutered singular of the Greek word meaning "first" inspired him to name this new essential component of the nucleus the proton. Rutherford was also thinking of Prout's use of the word "protyle," though. At the Cardiff meeting of the "British Association for the Advancement" of Science, starting on August 24, 1920, Rutherford gave a speech. In order to distinguish the positively charged hydrogen nucleus from the neutral hydrogen atom, Oliver Lodge requested that he suggest a new name at the meeting. Proton and prouton (named for Prout) were his early suggestions. Following Prout's word "protyle," Rutherford proposed that the hydrogen nucleus be given the name "proton," which the meeting adopted. In scientific literature, the term "proton" first appeared in 1920. Coulomb MeaningThe coulomb (C) is the common unit of electrical charge recognized by the "International System of Units" (SI). The amount of power a 1-ampere (A) current can carry in a second is known as the flux (s). 1 C is roughly equivalent to 6.24 x 1018 protons or electrons in terms of electrical charge. 6.24 quintillion particles are involved in this. According to the SI standard, the 'coulomb' is a derived unit, meaning it is created using any of the 7 basic units, in this case, the ampere and the second. Prior to 2018, the derivative units were built from the base units, which were the basis for the SI standard. The standard is now based on seven defining constants, from which all base & derived units can be formed. Nonetheless, the base & derived units have been kept in the SI standard due to how well-established they are. 
The elementary charge, or electric charge that a single electron or proton carries, is one of the seven fundamental constants. 1.602176634 × 10-19 C is the elementary charge (e). Protons and electrons both have the same charge level. An electron has a negative charge, whereas a proton has a positive charge. This means that the elementary charge might be either positive (+e) or negative (-e). The standard fixes the coulomb at a precise number of electrons, protons that make up 1 C of charge, by adjusting the elementary charge at 1.602176634 × 10-19 C. To determine that amount, apply the formula below: Q = n ⋅ e The letters Q and n represent the number of protons or electrons and the number of coulombs of charge, respectively. The amount of particles in a coulomb could be estimated by dividing both sides of the equation by e. Q = n ⋅ e Q / e = (n ⋅ e) / e n = Q / e (after Q / e = n is reversed) n = 1 C / (1.602176634 × 10-19 C) n = 6.24150907 × 1018 C Since the goal is to determine how many particles make up a single coulomb, 1 C is used in lieu of Q in the fourth line, and the elementary charge constant is used in place of e. According to this computation, 1 C has a charge of about 6.24 × 1018 particles, which would be represented as follows: 6,241,509,070,000,000,000
Next TopicElectronic Bulb
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









