Blackbody radiationThe blackbody radiations describe the absorption and emittance power of a system or a body. A blackbody is a body that absorbs all the incident radiations, which means the radiations falling on it. It also emits radiation at a rate similar to the rate of absorption. The radiations thus emitted by a blackbody are known as blackbody radiations. The black bodies are generally good absorbers and emitters. Note: Black color is a good absorber of heat and light.ExampleLet's understand the concept of blackbody radiations with the help of an example. Consider two bodies A and B placed in a room of equal surface areas. Body A is a blackbody (painted with black color), and body B has polished surface. After some time, the two bodies will be at room temperature. The radiations are allowed to fall on both bodies. We know that these two bodies have equal surface areas; hence the radiations falling on both will be the same. 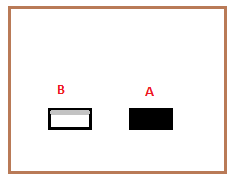
Body A, the blackbody, will absorb most of the radiations and reflect a very few of them. However, the polished surface always reflects. Hence, body B will reflect most of the incident radiations and absorb a few of them. It means that these two bodies work oppositely. Since the temperature is constant, body A will emit the radiations faster, while body B emits at a slower rate. Thus, the good absorbers of heat are also good emitters. A blackbody is often known as the ideal radiator because of its good emitting properties. Is the absorbing efficiency of a blackbody is 100%?We have read that a blackbody absorbs all of the incident radiations, representing 100% of the radiations. But, the above example depicts that it absorbs most of it but reflects a few. It is because 100% efficiency is only an ideal case, which is practically impossible. It reflects only a few radiations, i.e., only around 1%. What will happen if the light is enclosed in a box painted black?Let a box painted black be placed with a small hole that allows the radiations to pass. When the radiation falls, it goes inside the hole and keeps on reflecting from one surface to the other in a closed box. 
The radiations have little chance to escape from the hole because of the cone placed on the opposite side of the hole. After multiple reflections, it gets absorbed. It means that a few reflected radiations will not escape because of the pattern of the box. What is the behavior of real objects?The real object does not behave as a perfect blackbody, and emissions from such bodies are less than expected. The emissive power of a real object defines how a real body radiates emissions as compared to the blackbody, which is generally less. It depends on various factors, such as temperature, wavelength, and the angle of emissions. TheoriesThe theories described under the blackbody radiation are spectrum and blackbody. The theories of blackbody are already explained above, and let's discuss the spectrum. SpectrumSpectrum defines the color of the radiations emitted by a blackbody as the temperature rises. The emissions at the room temperature (about 25 degrees Celsius) are at the infrared region of the spectrum, which is not visible by the human eye. When the temperature passes 500 degrees Celsius, the emission starts appearing to the human eye. It is the low intensity light with a grayish shade. As the temperature rises, the radiations appear in the form of dull red color, yellow, and so on. At very high temperatures, the radiations appear a bluish-white in color. The color charts at various temperatures is shown below: 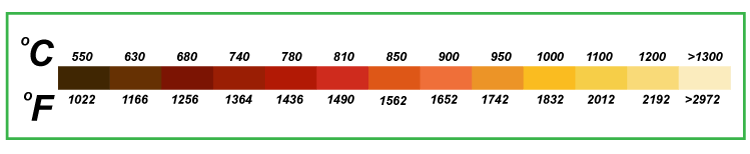
The Sun is also a type of blackbody with a surface temperature of 5505 degrees Celsius. The electromagnetic radiation given by the sun is generally infrared, ultra-violet, and visible light. The radiations emitted by a blackbody are also known as thermal radiations. The emissions are the process of the internal body, where the internal energy is converted into electromagnetic energy. The emissions of a blackbody depend on the temperature and the frequency distribution. Though, an ideal blackbody is not a practical example. But, the radiation enclosed in a black box with one hole is somewhat an example of an ideal blackbody. EquationThe equation of the blackbody radiation was discovered by a German physicist and the Nobel Prize winner named Max Carl Planck, Stefan- Boltzmann, and Wilhelm Carl Wien. Wien was also a German physicist. The laws described by them were named Planck's law of blackbody radiation, Stefan- Boltzmann's law of blackbody radiation, and Wien's displacement law of blackbody radiation. Let's discuss these laws and their equations in detail. Planck's law of black-body radiationThe Plank's law was discovered by Max Carl Planck. The discovery of the energy quanta (atoms and particles as a part of the quantum mechanics) won him the Nobel Prize in the branch of physics in 1918. Though he had made many discoveries and inventions in the field of physics, he was known for the development of quantum theory. Planck's equation is given by: 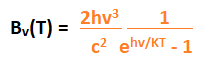
Where, 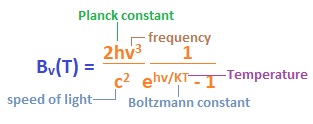
Bv(T) is the spectral radiance whose unit is calculated as power/(area x solid angle x frequency). Stefan- Boltzmann lawAccording to the Stefan- Boltzmann law, the discovery of the blackbody radiation is described in terms of the temperature. It is given by: j = σ T4 Where, J is the radiant emittance Sigma is the Stefan- Boltzmann constant T is the temperature It states that 'j (radiant emittance of the blackbody)' is directly proportional to the thermodynamic temperature, which is the temperature (T), defined in the field of thermodynamics. The radiant emittance of the blackbody is represented in terms of total energy radiated per unit surface area across all the wavelengths. Wien's displacement lawAccording to the Wien displacement law, the curves of radiations are different at different wavelengths, which are inversely proportional to the temperature. The blackbody spectrum for shorter wavelengths was already discovered by Wien before the Max Carl Planck. The equation is given by: Λ = b/T Wavelength at peak = Wien's displacement constant/Absolute temperature Where, Lambda (Λ) Wien's displacement constant (b) Absolute temperature (T) The Wien's displacement constant (b) is also known as the constant of proportionality. Blackbody radiation sourcesThere are various sources that radiate or emit blackbody radiations. The amount of radiations emitted by a source depends on the spectrum, temperature, emissive power, warm-up time, cooling time, and the stability. No single object is a perfect blackbody radiator. But, in carbon or graphite form, a material can be a good blackbody radiator. Let's discuss some of the most common sources, which are discussed as follows:
The sources such as electric heaters and light bulbs convert the electric energy into the light energy. The filament is heated and starts radiating energy in the infrared region. As soon as the filament is heated to its full energy, it reaches and glows in almost white light. When the voltage is low, such sources emit light white light due to insufficient energy. The stars appear in different colors due to the radiations emitted by them at different temperatures. It is because stars are also a type of blackbody. In the case of stoves, it behaves like a blackbody at high temperatures. Applications of blackbodyThe applications of the blackbodies are as follows:
Next TopicDew point
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









