Threshold EnergyThreshold energy is a concept that is often used in physics and chemistry to describe the minimum amount of energy required for a particular process to occur. It refers to the energy level required to initiate a specific reaction, such as the breaking of a bond or the excitation of an atom. The minimal amount of energy needed for a particle to pass through a wall or potential energy well is known as the threshold energy in quantum mechanics. The idea of activation energy, or the least amount of energy necessary for a chemical reaction to take place, is closely related to this one. Nevertheless, whereas threshold energy refers to the energy needed to start a reaction, activation energy specifically refers to the energy needed to achieve the transition state of a process. In the study of photochemistry-the interaction of light and matter-the idea of threshold energy plays a crucial role. The bare minimum of energy necessary to start a photochemical reaction is referred to as the threshold energy in photochemistry. Light's energy can be utilised to break bonds, excite electrons, or start other chemical events when it is absorbed by a molecule. Not all light wavelengths, though, have sufficient energy to start a reaction. Only light with a wavelength matching to an energy higher than the molecule's threshold energy will initiate a reaction. The concept of threshold energy is also important in the study of nuclear physics. In nuclear reactions, threshold energy refers to the minimum amount of energy required for a particular reaction to occur. This energy depends on the mass of the nuclei involved in the reaction, as well as the energy state of the nuclei. In some cases, the threshold energy for a nuclear reaction can be very high, and it may be difficult to achieve the necessary energy levels through conventional means. This is one reason why nuclear reactions are typically studied using high-energy particle accelerators. The term "threshold energy" is frequently used in chemistry to describe the bond-breaking process. An quantity of energy is used to hold two atoms together when they are joined. The molecule needs to be given a particular amount of energy in order to break this connection. The strength of the bond and the characteristics of the involved atoms are two variables that affect the threshold energy for bond breakage. In comparison to bonds between heavier atoms like carbon and oxygen, those between lighter atoms like hydrogen and oxygen have lower threshold energies. Types of Threshold EnergyThreshold energy is the minimum amount of energy required to initiate a certain process or reaction. It plays a crucial role in various fields of science and technology. Some of the important uses of threshold energy are:
Calculation of Threshold EnergyThreshold energy is the minimum amount of energy required to initiate a particular reaction or process. In other words, it is the energy required to break the bonds between the reactants and initiate a chemical reaction. The calculation of threshold energy is an essential step in understanding the kinetics of a reaction and designing experiments to study it. 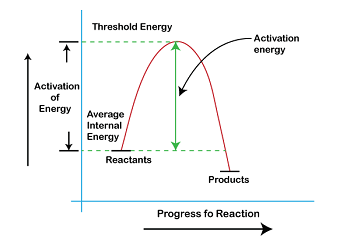
The threshold energy of a reaction can be calculated using a variety of methods, including computational methods and experimental methods. The choice of method depends on the specific reaction and the available resources. One common method for calculating threshold energy is the Arrhenius equation. The Arrhenius equation relates the rate constant of a chemical reaction to the activation energy, the temperature, and the frequency factor. The activation energy is the minimum amount of energy required to initiate the reaction, while the frequency factor is a measure of how frequently the reactants collide with enough energy to overcome the activation energy. The Arrhenius equation is given by: k = Ae(-Ea/RT) where k is the rate constant, A is the frequency factor, Ea is the activation energy, R is the gas constant, and T is the temperature. To calculate the activation energy, we can rearrange the equation as follows: ln(k/T) = ln(A) - (Ea/RT) By plotting ln(k/T) versus 1/T, it can be determined by the activation energy and from the slope of the resulting straight line. This method is known as the Arrhenius plot. Another method for calculating threshold energy is through transition state theory. Transition state theory is a framework for understanding chemical reactions that involves the concept of a transition state, which is a high-energy intermediate state that exists between the reactants and the products. In transition state theory, the rate of a reaction is proportional to the concentration of the reactants in the transition state. The concentration of the transition state can be calculated using the Boltzmann distribution, which relates the energy of the transition state to its probability of existence. The Boltzmann distribution is given by: P(E) = (1/Z) * e^(-E/kT) where P(E) is the probability of the transition state having energy E, Z is the partition function, k is the Boltzmann constant, and T is the temperature. The partition function is a measure of the number of ways that energy can be distributed among the particles in the system. It is given by: Z = ∫ e^(-E/kT) dE where the integral is taken over all possible energies. The threshold energy can be calculated using transition state theory by determining the energy of the transition state and the partition function. This method is particularly useful for reactions involving large molecules or complex reaction mechanisms. Experimental methods for determining threshold energy include temperature dependence studies and kinetic isotope effect studies. Temperature dependence studies involve measuring the rate of a reaction at different temperatures and using the Arrhenius equation to calculate the activation energy. Kinetic isotope effect studies involve comparing the rate of a reaction with different isotopes of the same element and using the difference in rates to calculate the activation energy. ConclusionIn summary, the calculation of threshold energy is a critical step in understanding the kinetics of a chemical reaction. There are several methods available for calculating threshold energy, including computational methods such as the Arrhenius equation and transition state theory, as well as experimental methods such as temperature dependence studies and kinetic isotope effect studies. The choice of method depends on the specific reaction and the available resources.
Next TopicAmpere Unit
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









