Thermal EnergyIntroduction of EnergyWe all have seen thermos, but have you ever wondered what keeps something hot and cold. Where does the energy inside an object and maintain the temperature? Well, first let's understand the term 'energy'. For doing every work whether it's simply kicked the ball or loading the heavy trunks on trucks, we all need energy. So, energy is 'the ability to do work'. There are various types of energy. Today we'll understand thermal energy, its types, characteristics, and applications. Also, in the end, don't miss the interesting facts about thermal energy: What is Thermal Energy?The energy that exists within the system or object and is responsible for its temperature, known as Thermal Energy. It is the internal energy that presents in the system, in the state of thermodynamic equilibrium. As we all have seen the flowing water. As the water flows from higher surface level to lower surface level till the water reaches an equal level. Similarly, in the concept of thermal energy, energy flows from high temperature to low temperature until equal temperature occurs. When the equilibrium occurs the flow of energy stops, that condition is known as Thermodynamic Equilibrium. So, in the state of thermodynamic equilibrium, (when both the system have the same energy), there will no work done happen. Characteristics of Thermal EnergyLet's see a few basic characteristics of thermal energy:
In solid-state, the molecular forces are too strong, so thermal energy is least and on the other hand, in the gaseous state, there is the least molecular force, so the thermal energy is highest.
Relation between Temperature and Thermal EnergyAs we discussed earlier, that thermal energy directly depends on the temperature of the system, so Thermal Energy ∝ Temperature Physicists take thermal energy to be equal to a product of K and T, where: Thermal Energy = KT Where, K= Boltzmann's constant (1.381 x 10-23m2kgs-2K-1) T= Absolute temperature The Unit of Thermal Energy is Joule. Heat Vs Thermal EnergyMost of the individuals get confounded between these two terms, Heat and Thermal Energy. It's basic to know the distinction between these wordings. Heat is the term we utilize in this setting to allude to the development of warm vitality from one thing or framework to another, with exchange being the vital word. Heat can be characterized as a stream of vitality from more smoking to a cooler protest or body due to the temperature distinction between those bodies. So warm is the exchange of vitality from higher temperature to lower temperature. Thermal energy is the energy put away inside a protest or framework as a result of molecule development. They're particular - thermal energy and heat. On the other hand, thermal energy is an internal property of a system that occurs before the flow of energy. 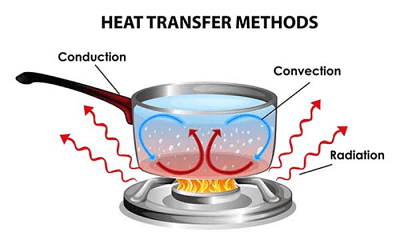
Types of Thermal EnergyA temperature slope is made when a body's constituent particles and atoms vibrate, coming about in an increment within the body's inner vitality (warm vitality). As a result, warm vitality is as often as possible categorized into distinctive categories based on how inner vitality is transmitted from one body to another within the shape of warm. There are three types of thermal energy or ways of transfer of thermal energy: 1. ConventionConvection is a phenomenon that occurs when heat is transported as a result of particle motion. It's most common in gases and liquids. The air becomes hot and transports heat energy when it is heated. 2. ConductionConduction occurs when heat is transferred due to the vibration of subatomic particles in their mean positions. When the two systems are close to each other, conduction occurs. Solids are better heat conductors than gases and liquids because their particles and molecules are closely packed. 3. RadiationRadiation is the process of transferring heat from one location to another via infrared electromagnetic waves. Radiative heat transfer does not necessitate the use of a medium. Hot objects radiate heatwaves in all directions at the speed of light, which are either absorbed or reflected by the item they strike. Applications of Thermal EnergyLet's take a look at some examples of thermal energy. This will you a broader perspective about the use of thermal energy and also, few examples from daily life scenarios: Solar EnergySolar means 'the Sun', so solar energy is the energy which we get from the Sun in the form of heat and light. Without this energy or radiation, there is no existence on our planet. The entire planet can be frozen in one night. So, why don't we should use this unlimited source of energy? Today, we are using this solar energy in generating electricity and running large machines. This resource is environmental friendly because it doesn't generate any smoke, hazardous gases, and waste for our ecosystem. Geothermal EnergyThe term Geo stands for the earth and thermal for the internal heat. It means Geothermal Energy is a form of energy found in the earth's crust. The temperature at the core of the earth is around 4000°C to 7000°C. When this heat comes near the earth's surface, it will become a source of energy. This energy flow from the deeper layer of earth towards the upper layer of earth. This heat is mainly generated in the core of the earth. The earth's crust is made up of molten rocks, carbon fuels and so on. Like solar energy, it is also not causing pollution in the environment. Heat Energy From the OceansThere is a massive source of thermal energy inside the oceans and seas. In oceans, if we go deeper from the shallow water, the temperature and pressure gradually increase. This happens due to exposure to the sun's rays. With technology, we can use that energy of seawater in several industries and machines. The technology which is used in the harnessing of thermal energy from the ocean is known as Ocean Thermal Energy Conversion (OTEC). Fuel Cell EnergyA fuel cell is also the source of energy. In the cell chemical reaction takes place on the electrodes, then it produces cathodes and anodes ions (negative and positive charges), and due to the movement of ions, a chemical reaction occurs and heat generates. This is can be used to increase energy efficiency. Melting IceIf we take a glass of water and added into the water. We will see that the water starts cool down as the ice will be melted. It means the temperature of water decreases as the temperature of ice increases. This happens due to the flow of thermal energy. Hot StoveTake the water in utensils and keep it on the stove for boiling. You'll see, after a few minutes the movement of the water molecules increases, and hence the temperature of water also increased. This happens due to the transmission of the thermal energy between the surface of utensils and the stove. Warm water in the bathtubIn winters, if you fill the bathtub with hot water. So, this warm water contains thermal energy inside it. When you go inside the tub, the transmission of thermal energy occurs between your cold body and warm water. So, it makes you feel comfortable in the winters. Facts about Thermal EnergyHere are a few interesting facts about thermal energy:
Next TopicIonization Energy
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









