What is a Chemical ChangeChemistry is a branch of science that deals with the study of chemicals as one of the main concepts. The elaborated description of chemical changes has become the primary goal of science in today's world. The study of chemicals and their interaction has played a significant role in discovering many new phenomena. The transformation of one substance into another form of the substance is known as a change, and a substance's ability to undergo a specific change in its chemical properties is known as chemical change. Chemical changes are also called chemical reactions. In a chemical reaction, one or more substances are changed into one or more new substances. For example, zinc is silver-grey naturally. At room temperature, when zinc is reacted with bright yellow-colored sulfur by providing some heat, a white-colored product known as zinc sulfate is obtained, as shown in the below image. Therefore, a chemical reaction leads to a chemical change. 1. Zinc
2. Sulfur
3. Zinc Sulphate
A famous scientist named Antoine Lavoisier has precisely defined changes as:"Nothing is lost, nothing is created, everything is transformed." In general chemistry, there are two types of changes-

Properties of Chemical Changes:A chemical change can be distinguished from a physical change based on its properties. Following are some properties or evidence of a chemical change
Types of Chemical Changes:The above evidence or properties of chemical changes are further categorized into three types of chemical changes:
These three terms are the pillar of chemical changes. Anything studied under chemical changes is directly or indirectly connected to these three changes, their applications, properties, and mechanisms. Every chemical change occurs only under suitable environmental conditions. Characteristics of Chemical Change:1) Evolution of Gas:Some chemical changes involve the evolution of gas while the reaction proceeds. The evolution of hydrogen gas characterizes a chemical reaction between zinc and sulphuric acid. Zn + H2SO4 -----> ZnSO4 +H2 Similarly, the reaction between zinc and hydrochloric acid is characterized by bubbles of hydrogen gas near the mouth of the flask. Zn + 2HCl -----> ZnCl2 + H2 2) Formation of Precipitation:Precipitation is an insoluble solid formed in a chemical reaction. The chemical reaction between copper sulfate and sodium hydroxide produces blue precipitation of copper hydroxide. CuSO4 + 2NaOH -> Na2SO4 + Cu (OH) 2 (Blue ppt) 3) State Change:All matter exists in three forms/states: solid, liquid, and gas. An example of a state change reaction is the combustion of candle wax. C2H5 + O2 ->CO2 + H2O + Heat and light Difference between Chemical Change and Physical Change:
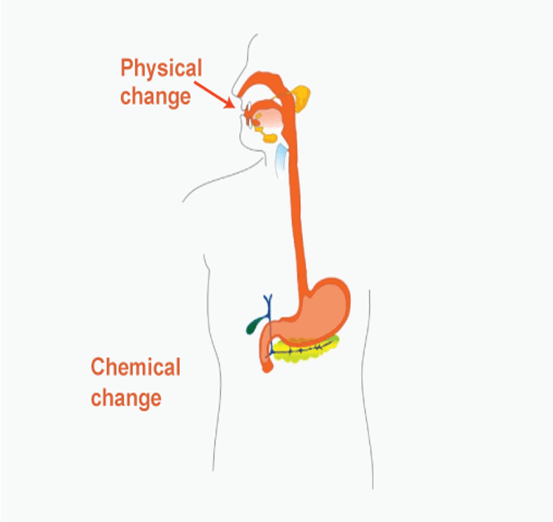
Chemical Changes from Everyday Life:1) Photosynthesis:Photosynthesis is a chemical reaction to convert carbon dioxide and water into glucose (food) and oxygen. Oxygen gas is evolved in this reaction, and an energy source compound called glucose is obtained. This chemical reaction takes place in the presence of sunlight. This is one of the most essential chemical reactions because it helps plants make food and provide oxygen to animals. 6CO2 + 6H2O + Sunlight -> C6H12O6 + 6O2 2) Aerobic Cellular Respiration:Unlike photosynthesis in aerobic cellular respiration, glucose molecules combine with oxygen molecules to release carbon dioxide and water molecules. The Equation for aerobic salary respiration is- C6H12O6 + 6O2 ->6CO2 + H2O + Energy The energy release is in the form of ATP. Generally, 37 Adenosine triphosphate molecules are released in one cycle of aerobic cellular respiration. 3) Metathesis:Metathesis is a chemical reaction between vinegar and baking soda to produce carbon dioxide and water bubbles of carbon dioxide gas are produced during the chemical reaction. It is a multi-step reaction usually performed while baking. 4) Soap and Detergent Reaction:Cleaning Agent soap and detergent clean through a chemical reaction. 5) Electrochemistry:The conversion of chemical energy into electrical energy is known as electrochemistry. Batteries use electrochemistry or redox (reduction Oxidation) reactions to convert chemical energy into electrical energy. 6) Anaerobic respiration:Anaerobic respiration is the process of Adenosine triphosphate synthesis by the cells in the absence of oxygen example, anaerobic respiration by yeast and bacteria to produce alcohol. Sometimes it is difficult to determine whether a change is chemical or physical. For example, when two or more metals are mixed to form an alloy, the properties of an alloy are entirely different from the metal it is formed for. Like brass, a standard alloy is made of copper (60%) and Zinc (40%), but brass properties neither match copper nor Zinc. Even though the properties of alloys (brass) differ from their constituent metal (copper and Zinc), it is not a chemical change because alloy and metal are not chemically bonded. As a result, brass represents physical change. Conclusion:Every event essential to life in the universe is because of chemical changes; it is because of the chemical reaction that human beings reproduce, grow, heal, digest, etc. The reason behind the growth of plants and the colour of fruits and flowers are all because of chemical reactions. Chemical changes are not only chemical reactions but also biological processes. The heart pumping and our ability to think are all chemical processes in the body. Chemical changes correlate chemistry to the biology of humans. Inorganic and organic changes deal mainly with reactions, whereas biochemical changes deal with biological processes. Hence, a particular set of conditions is required for a chemical change to occur. |
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share