Classification of Elements | Modern Periodic TableThe matter in our surroundings is made of basic units called elements. In around 1800, only 31 elements were known. Later 63 more elements were discovered over time with an advancement in technology. So, as the number of elements increased, there was a need to classify elements periodically. At present, there a total of 118 elements out of which 93 elements are found naturally and the rest were created in laboratories. It was not easy to study these elements separately without establishing a relationship between them based on their properties. So, these elements are organized or classified into different categories or group based on similarities and differences and periodicity in their properties. In the classification of elements, the elements are arranged in groups which are known as periods, so this classification system is known as the periodic classification of elements. History of classification of elementsMany scientists tried to classify elements in the past by giving different theories some of which are described below; Dobereiner's Triads: Around 33 elements were known when Dobereiner gave his theory for the classification of elements. He arranged the elements in groups of three or as triads in such a way that the atomic weight of the element at the middle was the arithmetic mean of the other two elements. However, his theory was not accepted as only 9 elements could be arranged or classified as triads based on his logic of arithmetic mean. Other available elements did not follow his logic when arranged in triads. Newland's Octaves: In 1864 Newland introduced his theory to classify the total number of elements present at that time. It was also called the law of octaves. In his classification, he arranged the element in order of increasing atomic mass and found that every eighth element in a series of element resembles the first element in many ways. Newland's theory was like octaves, which are found in music where every eighth note is the same as that of the first one. So, this theory was called Newland's Laws of Octaves. Mendeleev laws: In 1869, Mendeleev, Russian chemist, provided a systematic method for the classification of elements. His classification system was simple and effective and thus laid the foundation for the modern periodic classification of elements that is used as of now. During Mendeleev's time, only 63 elements were discovered. He classified or arranged the elements based on their physical and chemical properties. He reacted all the available 63 elements at that time one by one with hydrogen and oxygen and noted down their physical and chemical properties on different cards. After reacting the elements and noting their properties on cards, he arranged the cards in the order of increasing atomic masses on the wall. After arranging the cards in the form of a table some empty spaces were left in the complete table. Mendeleev suggested that these empty spaces indicate that there are elements that are yet to be discovered, so, he said that these empty spaces would be occupied by the elements that are not discovered yet. He even predicted the properties of these elements in advance based on the arrangement of elements in his table. His prediction was found true as when these missing elements were discovered their properties were almost similar as predicted by Mendeleev. Besides this, he also found that after arranging the elements in a systemic manner, the elements repeat their physical and chemical properties after certain intervals. Based on his findings and observation, Mendeleev introduced a law which he called the periodic law which states that the properties of elements are periodic functions of their atomic masses, which means their properties are repeated at regular intervals when arranged in the increasing order of their atomic masses. So, according to Mendeleev law, the elements are supposed to show similarities in the physical and chemical properties at regular intervals when they are arranged in the order of increasing masses. Although Mendeleev's periodic table was well-defined, few limitations were found in his table over time with the discovery of various new elements. The new elements could not be arranged in Mendeleev's periodic table as per the periodic law of Mendeleev. Limitations of Mendeleev's Periodic Tablei) Position of hydrogen element: The position of hydrogen was not acceptable in his table as some properties of hydrogen are similar to alkali metals and some are similar to halogens. ii) Position of isotopes: The position of isotopes was also not accurate. Isotopes are atoms of the same elements, they have the same atomic number, but the atomic mass is different. Mendeleev's table was based on the increasing order of atomic masses, but he assigned the same position to isotopes with different atomic masses instead of placing them at different positions. For example, isotopes of hydrogen occupied the same position in his table. iii) Presence of anomalous pairs: There were some elements, which were not present in the order of increasing atomic masses. For example, there were certain pairs of elements in which an element with more atomic mass was located before the element with less atomic mass such as Argon (Ar) with atomic mass 40 is located before the Potassium (K) with atomic mass 39. Similarly, Cobalt (Co) with atomic mass 60 comes before the Nickel (Ni) with atomic mass 59 in his table. Achievements of Mendeleev's Periodic Table
Later, at the beginning of the 19th century, a British physicist named Henry Moseley said that the elements will show periodicity if they are arranged in an order of increasing atomic numbers instead of atomic masses. He believed that the characteristics of elements are based on their atomic numbers not on their atomic masses. His belief gave rise to a new method for studying the periodicity of elements which is known as the modern periodic law. According to modern periodic law, the properties of elements are periodic functions of atomic numbers and not atomic masses which means the properties of elements occur periodically or repeat themselves periodically when elements are arranged in an order of increasing atomic numbers. This method allowed to predict the properties of undiscovered elements more precisely and helped in placing the newly discovered elements in the periodic table correctly. So, based on the Moseley's discovery, the Mendeleev's periodic law was modified, and atomic number was used to build the Modern periodic table. The modern periodic law was accepted widely, and it is the reason the modern periodic table that we use today is based on the modern periodic law. Structure of Modern Periodic TableThe modern periodic table contains 118 elements. It is divided into four blocks that include S block, P block, D block and F block. These blocks contain seven horizontal rows of elements, which are known as periods and 18 vertical columns of elements that are known as groups. Group 1 and 2 are placed in the S block, 13 to 18 groups are placed in the P block and the remaining groups from 3 to 12 are placed in the d block of the modern periodic table. 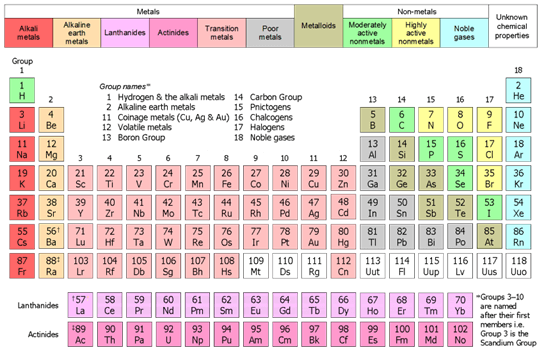
The elements are arranged in the modern periodic table based on their atomic number, so, the first box in the modern periodic table contains an element with atomic number 1 which is hydrogen, similarly, the second box contains Helium with atomic number 2, and the third box contains Lithium (Li) with atomic number 3 and so on. Besides this, the elements from an element with 57 atomic number to an element with 71 atomic number are kept in the upper series of F block. This series is called Lanthanide series as it starts with Lanthanum element with atomic number 57 and chemical symbol La. The lower or bottom row in the f block that lies below the Lanthanides series consist of elements that start from element with atomic number 89 to element with atomic number 102. This row or series of elements is called the Actinide series as its first element is Actinium with atomic number 89. So, the f block contains or consists of lanthanides and actinides series. The elements placed in the d block are called transition elements as they show transitional behaviour between s-block and p-block elements. Their properties are transitional (in-between) between metallic elements of s-block (which are ionic in nature and highly reactive) and d-block elements that are covalent in nature. Furthermore, the f-block elements are known as inner transition elements. The periodic table also contains metalloids that form a zigzag line in the periodic table. The properties of metalloids are intermediate between metals and non-metals or we can say that some of their properties are common with metals and some are in common with non-metals. The zigzag line made of metalloids separates the metals and non-metals. So, the zigzag line is bordered by metals on the left side of the periodic table and bordered by non-metals on the right side of the periodic table. So, in short, the S, D and F blocks of the periodic table contains metals, whereas, the non-metals and metalloids are present in the p-block of the periodic table. The first group of the periodic table contains alkali metals, the second group contains alkaline earth metals. The 18th group contains noble gases. Characteristic features of the modern periodic table
Next TopicPeriodic Trends in the Periodic table
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









