Ammonium-DichromateAmmonium dichromate, or ammonium dichromate (VI), is an explosive inorganic compound. With the chemical formula (NH4)2Cr207, It is a bright orange-red colored crystalline solid that readily ignites, burns, and produces a voluminous green residue. Ammonium dichromate is highly toxic and poses significant hazards, particularly when heated. It can also act as a strong oxidizing agent when mixed with or contaminated with combustible material. It dissolves when put into the water. Moreover, in this compound, as in all the chromates and the dichromate, chromium is in a +6 oxidation state, commonly called hexavalent chromium. It is a salt that comprises ammonium ions and dichromate ions, both at the same time. The ammonium dichromate is also sometimes popular as Vesuvian Fire. 
Structure of Ammonium DichromateThe chemical formula of ammonium dichromate is (NH4)2Cr2O7, indicating that it contains two ammonium ions (NH4+) and two dichromate ions (Cr2O72-). The structural arrangement of ammonium dichromate can be described as follows:
When these ions form ammonium dichromate, the structure can be visualized as two ammonium ions surrounding a central dichromate ion. The entire compound is held together by ionic bonds between the positively charged ammonium and negatively charged dichromate ions. 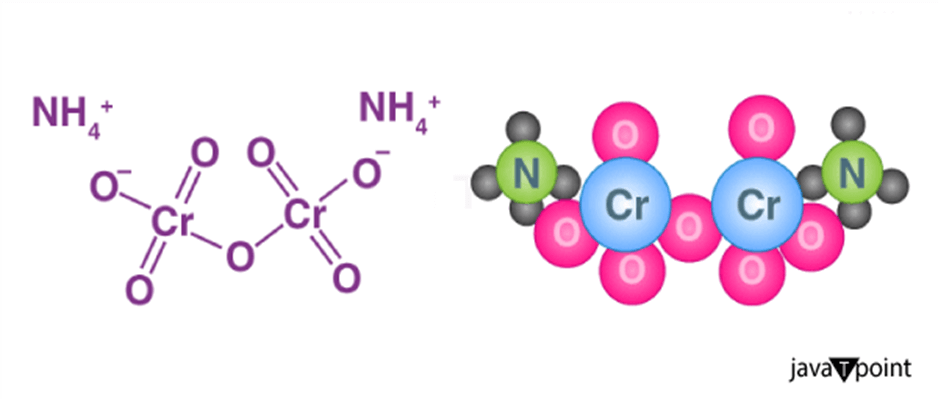
Physical Properties of Ammonium Dichromate
Chemical Properties of Ammonium Dichromate
Ammonium dichromate's thermal decomposition is also termed "volcano" or "chemical volcano". Thermal decomposition of ammonium dichromate is an exothermic reaction. During heating, ammonium dichromate produces visually striking effects. When the reaction is carried out, a dark green solid of (chromium (III) oxide) (Cr2O3) is left behind, while fumes of nitrogen gas and water vapor are released. The reaction can produce a distinctive "volcano" effect, where the solid mixture grows and expands while emitting gases. Professionals with proper safety precautions should only perform this reaction, as the decomposition of ammonium dichromate generates toxic fumes and can be dangerous if not handled correctly. It's also important to note that due to the toxicity and environmental hazards associated with ammonium dichromate, its use in demonstrations and experiments has been significantly reduced in recent years. Environmental Effects of Ammonium DichromateThe disposal of ammonium dichromate should be done following proper chemical waste disposal procedures, as it can harm the environment. Ammonium dichromate is toxic and poses health hazards if ingested, inhaled, or when it comes in contact with the skin. Chromium compounds, including hexavalent chromium found in ammonium dichromate, can be carcinogenic and can cause various health problems. The main hazards of ammonium dichromate are carcinogens and mutagens that are dangerous to the environment. Applications of Ammonium Dichromate1) Volcano Demonstrations: Ammonium dichromate was often used in educational demonstrations to simulate a volcanic eruption. When ignited, it decomposes exothermically, producing a large volume of green chromium (III) oxide (Cr203) ash that resembles the smoke and ash emitted during a volcanic eruption. This highly exothermic reaction produces a visual effect, which is a popular phenomenon in chemistry. 
2) Photography: In the past, ammonium dichromate was used in certain photographic processes. It could be combined with light-sensitive materials to create photosensitive plates for photo engraving and lithography. These processes were used in early printing methods; they have largely been replaced by more efficient and safer alternatives. 3) Pyrotechnics: Ammonium dichromate has been used in pyrotechnic compositions to produce vivid colors in fireworks displays. The heat generated during its decomposition can contribute to the emission of specific colors, enhancing the visual appeal of fireworks. 4) Chemical Reagent: Ammonium dichromate has been used as an oxidizing agent in various chemical reactions. For instance, it can oxidize alcohols to corresponding aldehydes and ketones. However, due to its toxicity and the availability of safer alternatives, its use as a general oxidizing agent has become less common. 5) As Pesticide: It is used as an approved pesticide and as a mordant for dyeing pigments. Health Hazards of Ammonium Dichromate1) Toxicity: Ammonium dichromate is considered highly toxic. Inhalation, ingestion, or skin contact with this compound can lead to serious health effects. Chromium compounds are known to have carcinogenic properties, meaning they have the potential to cause cancer with prolonged exposure. 2) Respiratory Irritation: Inhalation of ammonium dichromate dust or fumes can irritate the respiratory tract, leading to coughing, difficulty breathing, and a sore throat. 3) Skin and Eye Irritation: Direct skin contact with ammonium dichromate can cause irritation, redness, and chemical burns. Contact with the eyes can lead to severe irritation, redness, and potential damage to the cornea. 4) Allergic Reactions: Some individuals may develop allergic reactions upon exposure to ammonium dichromate. These reactions can range from mild skin rashes to more severe symptoms. Alternatives of Ammonium Dichromate1) Baking Soda and Vinegar for Volcano Demonstrations: A mixture of baking soda (sodium bicarbonate) and vinegar produces carbon dioxide gas and water, creating a visually similar effect to the volcanic eruption produced by ammonium dichromate. This reaction is safe and non-toxic. 2) Digital Imaging in Photography: Traditional photographic processes that involve ammonium dichromate are largely replaced by digital photography, which offers greater flexibility and reduced chemical hazards. 3) Safer Oxidizers in Pyrotechnics: Potassium perchlorate or potassium nitrate are commonly used as oxidizers in pyrotechnic compositions, providing controlled and vibrant colors without the toxic and carcinogenic properties of ammonium dichromate. 4) Other Oxidizing agents as Chemical Reagents: Safer alternatives for oxidation reactions include hydrogen peroxide (H2O2), potassium permanganate (KMnO4), and sodium chlorate (NaClO3), depending on the specific reaction requirements. ConclusionAmmonium dichromate is a chemical compound that has historically found application in various fields due to its unique properties. However, its usage has significantly declined due to its toxicity and environmental hazards. The compounds' distinct red and orange colour and exothermic decomposition reaction have been employed for education demonstrations, simulating volcanic eruptions. It has also been used in photography pyrotechnics and as an oxidizing effect in chemical reactions. Despite its historical uses, the safety concerns of ammonium dichromate are substantial. The compound is highly toxic, with the potential to cause respiratory irritation, skin and eye damage, allergic reactions, and even long-term health effects such as cancer due to its carcinogenic nature. Its environmental impact is also a cause for concern, as improper disposal can contaminate soil and water systems. As a result of these safety and environmental considerations, ammonium dichromate is being phased out in favor of safer alternatives. Substitutes with similar effects without the associated risks have emerged for applications such as volcano demonstrations, pyrotechnics, and oxidation reactions. In modern times, the advancement of digital imaging technology has reduced the demand for ammonium dichromate in photography. While ammonium dichromate once had its place in various applications, the broader understanding of its hazards has prompted a shift toward safer alternatives. This highlights the importance of prioritizing human health, safety, and environmental well-being in scientific and industrial endeavors. As we continue to advance, making informed choices that balance innovation with responsible practices is essential.
Next TopicIsotopes of Hydrogen
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










