When Should We Use R Gas Constant Value As 8.314 And As 0.0821?What is R gas constant?A fundamental constant in thermodynamics, the gas constant (denoted as R), is used to relate the characteristics of gases to one another. The ideal gas law, which specifies ,how perfect gases behave, has a reference to it. According to the ideal gas law, the relationship between an ideal gas's pressure, volume, and temperature is proportional to the number of moles (n) of gas that are present, with R serving as the proportionality constant. 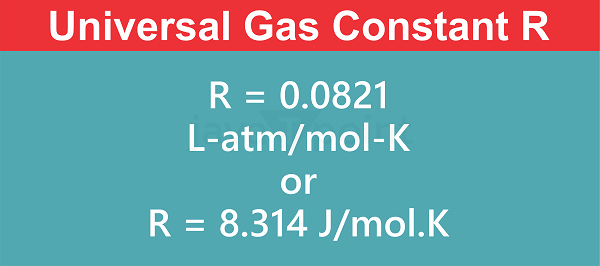
Depending on the chosen measurement method, R is expressed in a variety of units. J/(mol K) and L/(mol K) are the two most popular units. R stands for the gas constant in the former case in joules per mole-kelvin and the later case in liters-atmospheres per mole-kelvin. Other fundamental constants, such as Avogadro's number (Na) and Boltzmann's constant (k), can be used to determine the value of R. In non-SI terms, R is about equivalent to 0.0821 Latm/(molK), but in SI units, it is approximately equivalent to 8.314 J/(molK). When To Use R = 8.314 J/(mol�K)a. Energy Units R = 8.314 J/(molK) should be used when dealing with energy units measured in joules, such as for calculating the energy changes in a reaction or the heat transmitted during a process. Consistency in energy computations is made possible by this value. b. Molar Quantities When discussing molar quantities like the number of moles of a gas or the molar mass, R = 8.314 J/(molK) is employed. If the ideal gas law or other thermodynamic equations involving moles are calculated with this number, the units will cancel out correctly. c. Temperature Units R = 8.314 J/(molK) should be utilised when utilising Kelvin (K) as the temperature unit. Since Kelvin is an absolute scale with 0 representing no molecular motion, it is the favoured temperature scale in thermodynamics. R = 0.0821 L atm/(mol K): This ratio is utilised when converting between SI and non-SI units, especially when comparing pressure and volume measurements. In liters-atmospheres per mole-kelvin, this unit of R is defined. When To Use R = 0.0821 L�atm/(mol�K):a. Volume Units It is suitable to use R = 0.0821 Latm/(molK) when working with volume units in litres (L), such as for computing the gas density or measuring the volume of a gas. When litres are used as the volume unit, this value guarantees consistency. b. Pressure Units When using the atmospheres (atm) as a unit of pressure, R = 0.0821 L/(molK). Engineering and industrial applications where atm is the chosen pressure unit frequently use this value. c. Ideal Gas Law in Non-SI Units It is appropriate to use R = 0.0821 Latm/(molK) to keep the ideal gas law (PV = nRT) equation consistent while using non-SI units for pressure (atm) and volume (L). The choice of R value is influenced by the units that were used in the computation or problem-solving process, it is vital to remember this. In order to combine distinct equations or numbers accurately and meaningfully, it is essential to make sure the units are consistent. Through the ideal gas law, it is possible to connect the properties of gases to the gas constant, R. The units of measurement being utilised affect the value of R. When dealing with energy units, molar amounts, and Kelvin temperature, the value 8.314 J/(molK) is utilised in SI units. In non-SI units, especially when dealing with litres, atmospheres, and mol K, the value 0.0821 L atm/mol K is utilised. Applications of R Gas ConstantSome of the key applications of the gas constant. Ideal Gas Law The ideal gas law, which specifies how ideal gases behave, is not complete without the gas constant. PV = nRT is the equation for the ideal gas law, where P is pressure, V is volume, n is moles of gas, T is temperature, and R is the gas constant. In many branches of science and engineering, this equation is frequently employed since it enables us to link the basic characteristics of gases, such as pressure, volume, temperature, and number of moles. Gas Stoichiometry Gas stoichiometry, which examines the quantitative correlations between reactants and products in chemical reactions, depends heavily on the gas constant. It is easy to figure out how many reactants or products are involved in a reaction by using the ideal gas law and the idea of molar volume, which is the volume occupied by one mole of gas at a particular temperature and pressure. This is especially helpful in fields like chemical engineering and manufacturing where exact control over reactant quantities is essential. Thermodynamics The gas constant appears in a number of equations and relationships in thermodynamics. As shown by the equation U = nCvT, where Cv is the molar specific heat capacity at constant volume, it is used, for instance, to calculate the change in internal energy (U) of a system. The entropy (S) and enthalpy (H) variations of gases are also calculated using the gas constant. In the investigation of energy transfer and the choice of system parameters, these thermodynamic concepts are crucial. Gas Laws A key component of several gas laws, which explain the connections between various gas properties, is the gas constant. Gas laws include Boyle's law (PV = constant), Charles' law (V/T = constant), and Avogadro's law (V/n = constant). These principles, along with the ideal gas law, allow scientists and engineers to forecast outcomes and address gas-related issues under various settings. Real Gases While the ideal gas law presumes that gases behave optimally, real gases don't always behave that way, especially at high pressures and low temperatures. The Van der Waals equation, a variation of the ideal gas law that takes into account the intermolecular forces and the finite size of gas molecules, uses the gas constant. A more accurate illustration of actual gas behavior is provided by the Van der Waals equation. The gas constant is also incorporated into other equations of state, such as the Redlich-Kwong equation and the Peng-Robinson equation, to characterise non-ideal gas behaviour under various circumstances. Kinetic Theory of Gases According to the kinetic theory of gases, a gas's macroscopic characteristics are related to the movement and interactions of its constituent molecules. In several equations derived from the kinetic theory, like the one for the root mean square speed of gas molecules (vrms = (3RT/M)), where M is the molar mass of the gas, the gas constant is utilized. Comprehension of concepts like diffusion, effusion, and heat conduction requires a comprehension of these equations, which offer molecular-level insights into the behavior of gases. Energy Systems The field of energy systems and thermodynamic analysis both use the gas constant. It is used into equations that assess the effectiveness and functionality of various energy conversion systems, including power plants, internal combustion engines, and refrigeration systems. Engineers can assess and enhance the energy efficiency of such systems by factoring in the gas constant in these calculations. Ideal Solutions The gas constant plays a role in the study of ideal solutions, which are mixtures that exhibit ideal behavior similar to ideal gases. In the context of ideal solutions, the gas constant is used in equations like Raoult's law and Henry's law, which describe the behavior of volatile solutes in solvents. These laws find applications in areas such as chemical engineering, pharmaceuticals, and environmental science, where the behavior of solutes in solutions is critical to understanding their properties and interactions. Gas Chromatography The separation and analysis of mixtures of volatile substances is done using the commonly used analytical technique known as gas chromatography. In calculations involving gas chromatography, the gas constant is used to establish the link between temperature and retention time (the amount of time a substance spends in the chromatographic column). The components present in a combination can be identified and quantified based on their retention durations by knowing this relationship. Atmospheric Science In order to understand the behaviour and make-up of the Earth's atmosphere, atmospheric science is dependent on the gas constant. In equations that explain the characteristics of air, like the ideal gas law, it is used to calculate elements like air density, pressure, and temperature. To comprehend atmospheric processes, such as weather patterns, climate change, and the dispersion of air pollution, the gas constant is also used in simulations and models. Material Science The study of phase transitions and material properties uses the gas constant in material science and engineering. The Clausius-Clapeyron equation, which connects a substance's vapour pressure to its temperature during phase shifts like evaporation or condensation, uses this concept. Researchers can look into and forecast how materials will behave in various scenarios by adding the gas constant. Calibration of Instruments Different scientific instruments are calibrated using the gas constant. The gas constant, for instance, is employed to translate the measured values to the proper units in gas sensors and analyzers. It offers a fundamental conversion factor that links the electrical signals picked up by the instruments and the physical characteristics of gases, such as pressure and temperature, to the attributes of those signals. Educational Applications In science and engineering classes, one of the foundational ideas taught is the gas constant. Thermodynamics, gas laws, and other related concepts can all be understood using this as a foundation. Understanding the uses of the gas constant will enable students to comprehend and resolve issues concerning gases and their behavior, which are crucial in disciplines like chemistry, physics, and engineering.
Next TopicHow Can We Prepare 0.1 N HCl Solution
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










