Boric AcidBoric acid is a monobasic and weak Lewis acid of Boron. It is an inorganic compound with the chemical formula H3BO3. It is known by multiple names such as boracic acid, hydrogen borate, and orthoboric acid. It can exist as colorless crystals or as a powder, which is soluble in water. It is known for its antiviral, antifungal, and antiseptic properties. Boric acid is also found naturally in vegetables, grains, fruits and nuts. It is not toxic in small amounts that occur naturally. Chemical Structure of Boric AcidBoric acid is made of oxygen, boron and hydrogen atoms (H3BO3). Each molecule of boric acid has boron-oxygen single bonds. The boron is located at the center and is bonded to three hydroxide groups. Its molecular geometry is the trigonal planar. 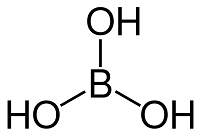
Boric acid crystals were made for the first time by Wilhelm Homberg in 1702. He prepared it by mixing borax and minerals acids with water. The water gets evaporated and crystals of boric acid are left behind, which were called "Homberg's salt'. Physical Properties of Boric Acid
Chemical Properties of Boric Acid
Uses of Boric Acid
Medical Uses of Boric AcidBoric acid is a natural antiseptic and anti-fungal agent. A solution of boric acid and distilled water can be used for wounds, minor cuts, rashes, acne, etc. It also reduces the itch caused by insect bites. Its solution in water can treat sore eyes, eye discharge and irritation. It can also treat vaginal yeast infection or candidiasis. It also helps cure athlete's foot, ear infections caused by fungi present in the swimming pool. It is also an ingredient of homeopathic medicine that is used to treat vaginal discharge and itching. The ingredients are vitamin E, and friendly bacteria. It also helps prevent foul food odor. A mixture of boric acid and talc can be applied to the inner side of footwear to treat smelly feet. It is also present in the urine sample bottles as a preservative. This is because it helps maintain the quality of the sample while it is shifted to the lab. Its addition reduces the chances of false results. Preparation of Boric AcidThe simple way of preparing boric acid is by reacting borax with a mineral acid such as hydrochloric acid as shown below; Na2B4O7.10H2O + 2HCl → 4H3BO3 + 5H2O + 2NaCl Or
Next TopicBenzoic Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









