Periodicity of Valence or Oxidation State of ElementsValencyThe term "valency" describes the number of chemical bonds that an element's or ion's atoms or ions can make with other atoms or ions. The atom's "valence" is another name for it. In other words, an element's valency is decided by the number of electrons in its atom's outermost shell, also referred to as the valence shell. The number of valence electrons that are available for bonding with other atoms or ions is determined by the valency of an element. Valence electrons are those that engage in chemical reactions and are found in the valence shell. For instance, an oxygen atom has a valency of 2 due to the fact that it has 6 electrons in its outermost shell, but needs 8 for a stable octet arrangement. Therefore, in order to finish its octet, it must gain 2 electrons, which it can do by creating 2 covalent bonds with other atoms. The valency of an element is used to anticipate the chemical formulae of compounds created by the combination of various elements and is a key factor in determining how it will react chemically with other elements. Types of Valency1. Electronegative Valency The quantity of electrons an atom needs to achieve a steady electron configuration is known as its electronegative valency. Since oxygen requires two more electrons to finish its outer shell and attain stability, it has an electronegative valency of 2. 2. Electropositive Valency The number of electrons that an atom can lose in order to create a positive ion is referred to as its electropositive valency. Since sodium can lose one electron to create the Na+ ion, it has an electropositive valency of 1. 3. Covalent Valency The quantity of electrons that one atom can share with another atom in order to create a covalent bond is known as the covalent value. For instance, because it can exchange four electrons with other atoms, carbon has a covalent valency of four. 4. Coordination Valency The amount of atoms or ions that can be coordinated around a main metal ion in a complex is referred to as the coordination value. Iron, for instance, has a coordination valency of 6 in a complex ion like [Fe(CN)6]4- because it can combine with six cyanide ions. 5. Oxidation Valency The term "oxidation valency" describes how many electrons an atom can acquire or lose during a chemical reaction. For instance, iron has a +2 oxidation valency in Fe2+ due to the loss of two electrons and a -2 oxidation valency in FeO due to the gain of two electrons. 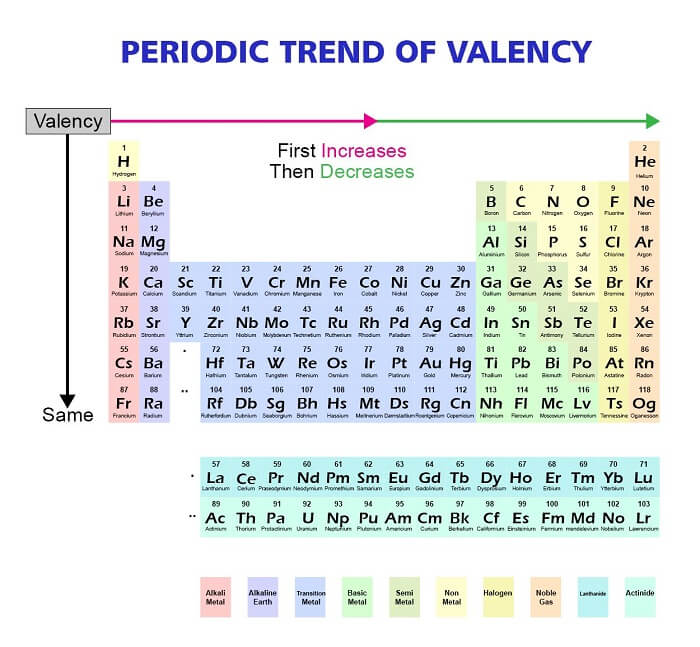
Oxidation StateOxidation state also referred to as oxidation number, is a metric for how much an atom in a chemical substance has been oxidised. It is an effective instrument for predicting how elements and compounds will behave chemically and is essential to many branches of chemistry, such as redox reactions, electrochemistry, and coordination chemistry. In general, an atom's oxidation state refers to how many electrons it has gained or lost in comparison to its neutral, uncombined form during a chemical reaction. When an atom gets electrons, it becomes reduced and has a lower oxidation state, whereas when it loses electrons, it becomes oxidised and has a higher oxidation state. Friedrich Wöhler, a German chemist, introduced the idea of the oxidation state in the middle of the 19th century. Since then, numerous chemists and scholars have improved upon and expanded upon this idea. It is now a cornerstone idea in chemistry and is frequently used in both theory and experimental research. The creation of sodium chloride by the reaction of sodium and chlorine is one of the most basic instances of an oxidation state (table salt). In this reaction, chlorine gets an electron to create a negatively charged ion while sodium loses an electron to create a positively charged ion (Na+) (Cl-). In this molecule, sodium has an oxidation state of +1, while chlorine has an oxidation state of -1. It can be more difficult to determine the oxidation state of each element in more complicated compounds. The oxidation state rules are a collection of guidelines and rules that can be used to determine the oxidation state of each element in a compound. These laws are founded on the understood conduct of various elements and their propensity to acquire or lose electrons during chemical reactions. The requirement that the total of the oxidation states of all the atoms in a neutral compound is zero is one of the fundamental principles for allocating oxidation states. For instance, the oxidation state of oxygen is -2 and that of hydrogen is +1 in water (H2O), so the total of the oxidation states is 0. The oxidation state of an element in a monoatomic ion is equivalent to the charge on the ion, which is another crucial principle. For instance, the oxidation state of sodium is +1 in the sodium ion (Na+), whereas the oxidation state of chlorine is -1 in the chloride ion (Cl-). Periodic Trends in Oxidation State of ElementsAn element's capacity to lose or gain electrons during chemical reactions is determined by the oxidation state of the element. It shows how many electrons an atom has added or taken away from its neutral condition. The periodic table offers a helpful framework for comprehending periodic patterns in oxidation states because it arranges elements in rows and columns according to their atomic number and electron configuration. The following are some major trends: 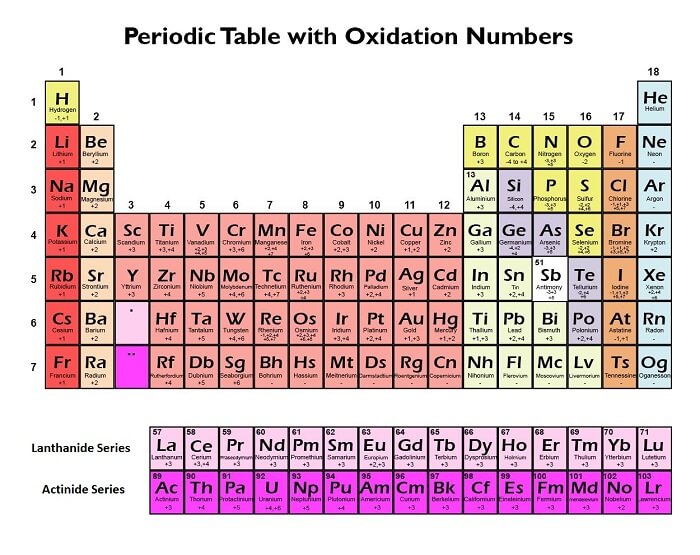
1. Group TrendThe oxidation state begins to rise as we descend a group in the periodic table. This is due to the valence electrons' greater ease of loss or gain due to their distance from the centre. A good example is the ns1 valence electron configuration of the alkali metals in Group 1 (such as lithium and sodium), which indicates that they have low ionisation energy and are more apt to lose their valence electron to form a +1 oxidation state. With a valence electron configuration of ns2, the alkaline earth metals in Group 2 (such as magnesium and calcium) have greater ionisation energy than the alkali metals but still have the propensity to lose their two valence electrons to form a stable ion. 2. Period TrendThe oxidation state typically rises until a certain point and then falls as we progress across a period in the periodic table. This is because it is more difficult to lose electrons when the amount of valence electrons remains constant while the nuclear charge increases. For instance, nonmetals on the periodic table's right side have the propensity to pick up electrons and adopt negative oxidation states. The halogens in Group 17 (including chlorine and fluorine) have a valence electron configuration of ns2np5, which indicates that they have a strong affinity for electrons and are apt to pick up an electron to create a -1 oxidation state. Group 16's oxygen family, which includes sulphur and oxygen, has a valence electron arrangement of ns2np4, indicating that although they have a lower affinity for electrons than halogens, they are still likely to acquire two electrons to achieve an oxidation state of -2. The metalloids and metals, on the other hand, have the propensity to lose electrons to create positive oxidation states as we move to the right side of the periodic table. For instance, depending on how many valence electrons they can lose, the transition metals in the centre of the periodic table can change their oxidation state. Due to their partly filled f orbitals, the actinides and lanthanides at the bottom of the periodic table frequently have high oxidation states. 3. Electronegativity TrendThe electronegativity of the elements rises across a period as we move from left to right. An atom's capacity to draw electrons to itself in a chemical bond is known as electronegativity. Because they are more apt to gain electrons, elements with higher electronegativity typically have higher oxidation states. For instance, fluorine, the element with the highest electronegative potential, frequently forms a -1 oxidation state. 4. Size TrendThe size of the atoms rises from top to bottom in a group. Larger atoms are more apt to lose electrons and develop positive oxidation states because they typically have lower electronegativity and ionisation energies. For instance, alkali metals tend to create a +1 oxidation state and have lower ionisation energy due to the larger size of their atoms. Overall, a number of variables, including the number of valence electrons, electronegativity, ionisation energy, and atomic size, can be used to describe periodic trends in oxidation states. These patterns can be used to forecast the reactivity and chemical characteristics of elements and their derivatives Norms for Determining Oxidation StateUnderstanding the chemical reactivity and characteristics of elements and compounds requires knowing their oxidation states, which is a crucial first step. Following are some recommendations for determining oxidation states:
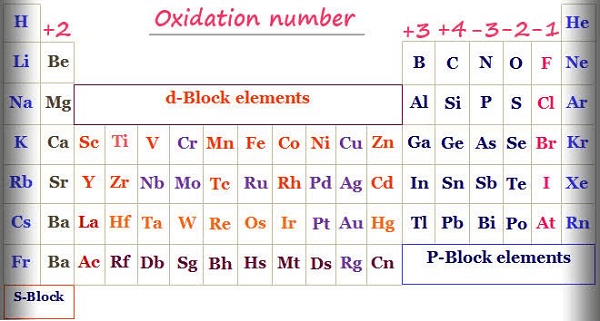
These are some general rules for determining oxidation states, but depending on the particular compound or circumstance, there may be exceptions and special instances. In order to analyse and forecast chemical reactions and properties, it is crucial to comprehend the underlying principles of oxidation state assignment. Steps to Determine the Valency of ElementsThe capacity of an element to combine with other atoms to create chemical compounds is measured by its valency. When an element makes a chemical bond with other atoms, the number of electrons that it can either acquire, lose, or share is represented. To find the valency of an element, you can use the following steps: 1. Determine the Number of Valence Electrons The electrons in an atom's exterior shell are known as valence electrons. The periodic table can be used to determine the number of valence electrons. The amount of valence electrons in an element is represented by its group number. For instance, Group 1 elements have a single valence electron, while Group 2 elements have two valence electrons. 2. Determine the Electron Configuration An atom's arrangement of electrons at various energy levels is called its electron configuration. How many valence electrons a material has can be determined in part by looking at its electron configuration. For instance, oxygen has a 1s2 2s2 2p4 electron arrangement, which means it has 6 valence electrons. 3. Determine the Number of Electrons Needed to Achieve a Stable Configuration The octet law, which is how most elements typically acquire eight electrons in their outermost shell, stabilises the majority of elements. But there are some exceptions, like hydrogen and helium, which can reach a stable configuration with just two electrons. 4. Calculate the Valency Calculate an element's valency by deducting the number of valence electrons from the total number of electrons required to attain a stable configuration. The valency is equivalent to the difference if the outcome is positive. The valency is the absolute worth of the difference if the outcome is negative. Here are some examples: Sodium (Na): The topmost shell of sodium contains one valence electron. It must lose this electron in order to attain a stable configuration. Therefore, sodium has a valency of 1. Oxygen (O): In its outermost layer, oxygen has six valence electrons. It requires gaining two electrons in order to reach a stable configuration. Therefore, oxygen has a valency of -2. Aluminium (Al): Three valence electrons make up the topmost shell of aluminium (Al). It requires losing three electrons in order to reach a stable configuration. As a result, aluminium has a valency of +3. In order to determine an element's valency, you must first identify its electronic structure, count the number of valence electrons, and compute how many electrons are required to produce a stable electron configuration. The number of electrons the element must acquire or lose to achieve a stable configuration is then equal to the valency of the element. Rules Useful for Determining Oxidation States of ElementsPrinciples that can be used to determine an element's oxidation state
Valency of First 20 ElementsHere are the valencies of the first 20 elements:
Note that some elements, like nitrogen and phosphorus, have more than one valency, depending on the specific compound they are in. Additionally, the valency of an element can change depending on the conditions under which it is reacting.
Next TopicBase Meaning
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









