What is valencyThe number of electrons gained or lost by an atom to complete its outermost shell is known as valency. The atoms become stable by completing their outmost shell or octet (8 electrons in the outmost shell). So, they tend to combine with other atoms or take part in chemical reactions in which they may give or lose electrons to other atoms or may take or gain electrons from other atoms to achieve a stable state by completing their octet. So, the reason for bond formation or a chemical reaction that takes place between atoms is that atoms are always in a constant struggle to reach the stable state by completing their outermost orbit or shell. For example, if an atom has one shell, the stable state is achieved when it has two electrons in it. Similarly, if an atom has two or more shells or orbits, the stable state is achieved when the outermost shell contains eight electrons. For example, Hydrogen has only one electron in its atom in the outermost shell. So, it has to somehow add one more electron to its outermost shell or orbit to complete electrons in its outermost orbit. Similarly, the oxygen has electronic configuration 2, 6. So, it has 6 electrons in its outermost shell or orbit. Therefore, it will need two more electrons to complete the octet. Similarly, Magnesium with atomic number 12 will have electronic configuration 2, 8, 2. So, it will need 6 electrons to complete the octet. But, it is not easy for an atom to give 6 electrons to Mg. So, Magnesium will complete its octet by donating or giving its 2 valence electrons to atoms of other elements. Let us take an example where elements are forming molecule or compound to understand it. In a compound of NaCl, a molecule of NaCl contains one atom of Sodium (Na) and one atom of Chlorine (Cl). The electronic configuration of Na is 2, 8, 1. While, the electronic configuration of Chlorine is 2, 8, 7. Now, from the electron configuration of these two atoms, it is clear that sodium is in a position to donate one electron and chlorine would like to take one electron to complete its octet. So, chlorine gets one electron from sodium and sodium loses or give one electron to chlorine as it was the easiest way for it to achieve stability or to complete its octet. So, in this case, the valency of both Sodium and Chlorine is 1. 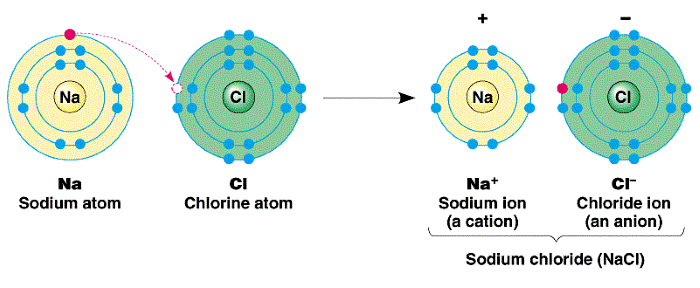
This give and take of electrons help in the formation of the Sodium Chloride molecule. But, it does not mean that only the elements with this condition combine to form a bond. Let us take another example of the water molecule to understand this, e.g. H2O. The valency of hydrogen with atomic number 1 is one, whereas, the valency of oxygen with atomic number 8 (2, 6) is 2. Here, both need electrons. So, in this case, they will share electron with each other to form one molecule of water and to complete their octets. For example, two atoms of hydrogen will combine with one oxygen atom as shown in the below image. Such bonds which are formed by sharing electrons are called covalent bonds. 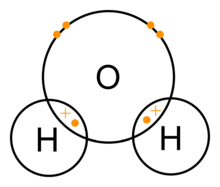
However, there are also elements that have a stable state and don't need to react with other atoms to achieve stability. For example, Helium (2), Neon 10 (2, 8) and Argon (2, 8, 8) have atoms with stable state or complete octet. So, we can say that valency is the combining capacity of an atom to react with other atoms. It also determines how many electrons will participate in bond formation and whether an atom will gain or lose electrons. Let us see how to determine the valency of an atom or element; How to find the valency of an Element?Valency is associated with the loss or gain of electrons and is different from the total number of electrons in an atom. For example, the atomic number of Sodium is 11, so, it has 11 electrons but its valency is 1. As its electronic distribution is (2, 8, 1), so, it is easy for it to lose 1 electron than gaining 7 electrons to achieve stability or complete its octet. So, its valency is 1. Similarly, the valency of oxygen with atomic number 8 and electronic figuration (2, 6) is 2 as it is easy for it to gain two electrons to complete its octet. On the other hand, atoms like Fluorine with electronic configuration (2, 7) tends to gain one electron instead of losing all of its 7 electrons as it is easy for it to gain one electron than losing seven electrons, So, its valency is 1. Besides this, there are also elements in the periodic table whose valency is zero such as noble gases like helium (He), neon (Ne), argon (Ar) as they have 8 electrons in their outermost shell or their octet is complete. So, they do not react with other atoms or elements or they are said to be the least reactive and due to this reason they are also known as inert gases. Furthermore, the charge on an atom when it becomes an ion after losing or gaining electrons exists due to valency. For example, the charge on sodium ion is -1 as it loses one electron in a reaction to gain an ideal electronic configuration (2, 8) or to have a completely filled outermost shell or orbit. Similarly, the charge of Magnesium ion when Magnesium loses 2 electrons is +2 (Mg2+). Its electronic configuration is (2, 8, 2), so, it tends to lose 2 electrons. Types of Valency Atoms combine to complete their octet, which results in the formation of compounds which can be ionic compounds or covalent compounds. Accordingly, valency can be of two types as described below; ElectrovalencyThe atoms that form ionic or electrovalent compounds show Electrovalency. So, Electrovalency refers to the number of electrons lost or gained by an atom to achieve a stable state or complete its octet. The atom which loses electrons form positive ions (cations), so their valency is called positive electrovalency. The atoms which gain electrons form negative ions (anions) so, their valency is called negative electrovalency. So, electrovalency is the valency in Ionic (electrovalent) compounds or Electrovalent Compounds which are generally formed between metals and non-metals. For example, sodium chloride (NaCl) where sodium is metal and chlorine is non-metal. The chemical bond between the atoms of sodium and chlorine in the Sodium Chloride compound is formed by the transfer of electrons from Sodium to Chlorine. Sodium loses one electron and Chlorine takes one electron. So, the electrovalency of both sodium and chlorine is 1 as Na is losing 1 electron and Cl is gaining one electron as shown below;
CovalencyIt refers to the number of electrons shared by atoms or elements while forming a covalent compound. In covalent compounds, the chemical bond is formed between non-metals. Atoms one non-metal combines with atoms of other non-metals to form molecules of covalent compounds. In this case, the atoms do not gain or lose electrons instead they share electrons. For example, in Methane (CH4), one atom of carbon, which needs four electrons to complete its octet, combines with four hydrogen atoms as each hydrogen atom needs one electron to complete the required 2 electrons in its first orbit. So, in a molecule of methane, one carbon atom is attached to four hydrogen atoms through four single covalent bonds as it is sharing its four electrons with four hydrogen atoms. So, the co-valency of carbon is four in methane. One hydrogen atom is attached to Carbon through one covalent bond so the co-valency of hydrogen is 1. What is variable valency?There are also elements that show more than one type of valency which is known as variable valency. Such elements show different valencies in different compounds. For example, transition elements like iron, mercury and copper show different valencies. Iron's valency is 2 in FeSO4, whereas, in FeCl3 the valency of Fe is 3. Copper shows two different valencies 1 and 2. Mercury also shows two different valencies 1 and 2. Besides this, if we know the valencies of elements, we can write the chemical formula of a molecule or a compound as shown below; Chemical formula of carbon tetrachloride.The valency of carbon is 4 and the valency of chlorine is 1. In this case, sharing of electrons will take place between one carbon atom and four chlorine atoms to complete their octets. So, there will be one carbon atom and four chlorine atoms in the chemical formula of carbon tetrachloride, e.g., CCl4. Chemical formula of magnesium chlorideThe valency of magnesium is 2 and chlorine is 1. In this case, give and take of electrons will take place to complete the octet. Magnesium will give 2 electrons to 2 Chlorine atoms. Each chlorine atom gets one electron. So, one Magnesium atom and two chlorine atoms will form the chemical formula of magnesium chloride, e.g. MgCl2. What are valence electrons?The total number of electrons present in the outermost orbit or energy shell of an atom is called the valence electrons. For example, the electronic configurator of Magnesium is 2, 8, 2. It means K orbit has 2 electrons, L orbit has 8 electrons and M orbit has 2 electrons. So, there are two electrons in the outmost orbit of Magnesium. So, it has 2 valence electrons. Similarly, Chlorine (Cl) whose electronic configuration is 2, 8, 7, has 7 valence electrons. The outermost shell that contains the valence electrons is called the valence shell. 
Next TopicMolecule
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










