Metals and Non-Metals
All the substances or materials around us can be metal or non-metal. Both metals and non-metals are elements. Let us discuss metals and non-metals one by one!
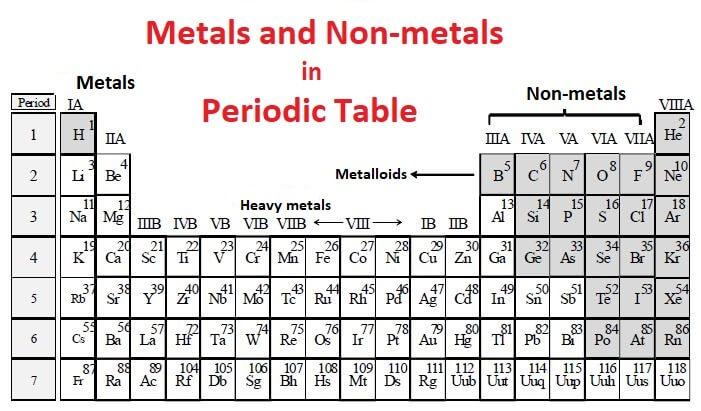
What are metals?
Most of the elements in the periodic table are metals, which include alkali metals, alkaline earth metals, transition metals, lanthanides and actinides. The metals are present on the left side of the periodic table. They are separated from the non-metals by a zig-zag line that passes through metalloid elements such as boron, silicon, arsenic, etc. Metalloids or semimetals show properties intermediate between metals and non-metals. The non-metals are present on the right side of the periodic table.
Metals are generally formed below the Earth's surface. They are inorganic substances or minerals which means they are made of non-living substances. More than 75% of elements present in the periodic table are metals, which means most of the periodic table is occupied by metals.
In the Earth's crust, they are found in the form of metal ores in which they can be combined with each other or with other elements. However, some metals like copper, gold, silver and platinum are found in Free State as they do not tend to react with other elements or metals. Besides this, they are found in the rocks washed by surface water, in groundwater and in atmospheric dust.
Physical properties of metals
- They are lustrous or have shining surfaces.
- Good conductor of heat and electricity.
- The density and melting point of metals is very high.
- They are malleable which means they can be hammered into flat sheets. The metals sheets are widely used in the manufacturing of aircraft, automobiles, utensils, etc.
- They are ductile which means they can be drawn into wires, for example, copper wires are made of copper metal.
- They are found in solid state at room temperature except for mercury.
- Their thin sheet is opaque; you cannot see through them.
- They are sonorous which means they make a bell-like sound when hit.
- The metals can be polished to increase their shining or to give them a shining surface.
Chemical properties of metals
- They have 1 to 3 electrons in the outer shell of their atoms.
- They tend to lose electrons easily to obtain a stable electronic configuration.
- They readily undergo corrosion, e.g. damaged by rust.
- They form oxides that are basic in nature. The metal oxides are formed by burning metals in the presence of oxygen in the air. The highly reactive metals react vigorously when they are burnt in the presence of oxygen. For example, a magnesium strip when burned in the air produces magnesium oxide which forms magnesium hydroxide when dissolved in water.
- They have low electronegativities (attraction for electrons).
- Metals are excellent reducing agents as they can lose electrons easily to reduce cations or positively charged atoms of other metals.
- Metals are used to form alloys by combing metal with other metals or non-metals.
- The highly reactive metals like potassium and sodium (K and Na) are stored in oil as they tend to react with air.
- The less reactive metals like gold, platinum, and silver do not tarnish or corrode. They remain shiny and lustrous.
- They produce metal oxide and hydrogen gas when reacting with water. The metal oxides being soluble in water form metal hydroxide. For example, sodium reacts rapidly with water and oxygen to form NaO.
- Metals produce hydrogen gas when reacting with acids. For example, when Zinc (Zn) reacts with hydrochloric acid, it produces zinc chloride and hydrogen gas.
- When they react with bases, metal salts and hydrogen gas is produced.
- A more reactive metal displaces less reactive metal from its salt solution. This reaction is called a displacement reaction. For example, iron being more reactive than copper displaces copper from its salt solution as shown in the below reaction;
CuSO4 + Fe → FeSO4 + Cu
Uses of metals
Metals are known for their durability, strength and resistance to wear and tear. Since ancient times, metals have been used for various purposes. Let us see some of the major uses of metals in different fields.
- In the construction industry: Metals are widely used in the construction industry. Without metals, you cannot imagine the construction of buildings. For example, iron and steel are the main materials required in constructing homes, buildings, etc.
- In Electronics: As metals are good conductors of electricity they are widely used in the electronic industry. They are used to make wires and parts of machines and other devices. For example, they are used in TV, fridge, mobiles, computers, and more.
- In medicine: Metals are also an important part of our body. For example, iron is found in red blood cells and iron deficiency causes anaemia. So, metals are used in medicines to treat deficiencies and sickness. Besides this, metals like magnesium, calcium and aluminium are used as antacids. Most of the equipment in hospitals used to treat patients are also made of metals.
- In Machinery: Metals are widely used in the production of machinery required for farming, automobiles, household works, etc. For example, tractors, combines, vehicles, airplanes, rockets, mixer grinder and kitchen utensils all are made of metals.
- Jewellery and ornaments: Almost all jewellery is made of gold, silver and platinum metals. These metals are durable and maintain their shine forever, so they have high economical value and are thus used in making jewellery.
Other uses of metals
- Metals are used to make locks, safes, doors, etc.
- They are used in the manufacturing of weapons, bullets, explosives, etc.
- Mercury is used in thermometers.
- Aluminium foils are used in packaging medicines, food, cigarettes, etc.
What are non-metals?
Non-metals are the elements located on the right side of the periodic table except for hydrogen which is the only non-metal that is located on the left side of the periodic table.
Physical properties of non-metals
- Non-metals are not malleable, which means they cannot be hammered into thin sheets. However, they are brittle as they break into pieces when hammered.
- They are not ductile, which means they can be drawn into wires.
- Poor conductor of heat and electricity.
- They do not have a lustrous surface, so, they are dull in appearance.
- Tensile strength is low so they cannot withstand heavy weights.
- They are not sonorous, which means they do not make a sound when hit.
- They are soft which means they can be cut easily.
- They have a low melting point except for diamond, which is a non-metal with a high melting point.
Chemical properties of non-metals
- They generally have 4, 5, 6 or 7 electrons in their outermost or valence shell. So, they tend to form anions by gaining electrons as it allows them to have a stable electronic configuration.
- They form non-metallic oxides when they react with oxygen. These oxides are acidic in nature. For example, when sulphur, a non-metal, reacts with oxygen, it forms sulphur dioxide, which produces sulphurous acid when reacts with water. The reaction is shown below;
S + O2 → SO2
SO2 + H2O → H2SO3
- An aqueous solution of non-metal oxide changes blue litmus paper into the red.
- They do not react with dilute acids, however, they can react with concentrated acids. For example;
C + 4HNO3 → CO2 + 4NO2 + 2H2O
- They react with strong bases at high temperatures. In this reaction, hydrogen gas is not produced. For example
4S + 8NaOH → Na2SO4 + 3Na2S + 4H2O
- A less reactive non-metal is displaced from its salt solution by a more reactive non-metal. For example ,see the below reaction;
2KI + Br2→ 2KBr + I2
In the above reaction, Bromine being more reactive than Iodine displaces iodine from potassium iodide compound.
Uses of non-metals
- Nitrogen is widely used for the preparation of ammonia, nitric acid and fertilizers.
- Chlorine is an important disinfectant. It is used for the purification of water.
- Hydrogen is used in the rocket fuel.
- Carbon in the form of graphite is used to form pencils.
- Sulphur is used to form sulphuric acid.
- Oxygen is essential for life. We not only need it for breathing but also for medical use and for combustion.
- Sulphur also has medicinal properties and is used as an important component in various chemical solutions.
Difference between metals and non-metals
| Based on |
Metal |
Non-metal |
Exceptions |
| Malleability |
Metals can be hammered. They can be changed into sheets. |
Non-metals cannot be hammered into sheets. |
- |
| Ductility |
They can be drawn into wires such as copper wires. |
They cannot be drawn into wires. |
|
| Brittle |
They are not brittle. |
They are brittle so they break into pieces when hit. |
|
| Conductor |
They are good conductors of heat and electricity such as copper, gold, etc. |
Poor conductor of heat and electricity. |
Diamond is a non-metal, but a good conductor of heat. Graphite is a non-metal still is a good conductor of electricity. |
| Lustre |
Metals are lustrous; they have a shiny surface. |
They are non-lustrous. Their surface is dull. |
Diamond and iodine non-metals are lustrous. |
| Strength |
They have high tensile strength. |
Their tensile strength is low. |
Although sodium and potassium are metals, their tensile strength is low. |
| Sonority |
They are sonorous; the sound is produced when they are hit. |
They are not sonorous; they don't make a sound when struck. |
|
| Hardness |
They are hard such as iron, aluminium, copper, etc. |
They are soft. |
Although sodium and potassium are metals, they are soft. Diamond is a non-metal but it is very hard. |
| Reaction with oxygen |
Metals form metal oxide when reacting with oxygen, e.g., 2Mg + O2 → 2MgO |
Non-metals form non-metal oxide by reacting with oxygen, e.g., S + O2 → SO2 |
|
| Reaction with water |
They form metal hydroxide and hydrogen gas by reacting with water, e.g., Na + H2O → NaOH + H2 |
They do not react with water |
|
| Reaction with acid |
They form metal salt when reacting with acid, e.g., Mg + 2HCl → MgCl2 + H2 |
They react with only concentrated acid, e.g., C+ 4HNO3 → CO2 + 4NO2 + 2H2O |
|
| Reaction with base |
They form metal salt and hydrogen gas by reacting with base, e.g., Al + NaOH → NaAlO2 + H2 |
They react with strong bases but do not produce hydrogen gas in this reaction, e.g., 4S + 8NaOH → Na2SO4 |
|
| Nature of oxides |
Their oxides are basic in nature. |
Their oxides are acidic in nature. |
|
| Litmus paper |
Metal oxides turn red litmus paper into the blue. |
Non-metal oxides turn blue litmus paper into the red. |
|
| Reactions of oxides with water |
Their oxides react with water to form hydroxides, e.g., MgO+H2O → Mg(OH)2 |
Their oxides react with water to form acids, e.g., SO2 + H2O → H2SO3 |
|
|

 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now










