Base MeaningBase: A base is a chemical substance that can neutralize the effect of an acid. A basic substance is soapy or slippery in nature. It has the ability to turn red litmus paper to blue. Bases can react with an acid to form salt and water and the reaction is called neutralization reaction. A base has a pH always greater than 7. A few examples of bases are sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)2), ammonia (NH3), soaps, toothpaste, etc. There are three major theories and definitions of a base, and these are:
1. Arrhenius's theory of bases: In 1887, the famous Swedish scientist Arrhenius described bases as the chemical substances which release OH-(hydroxide) ions in an aqueous solution. Sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)2), ammonium hydroxide (NH4OH), etc., are a few examples of Arrhenius bases.
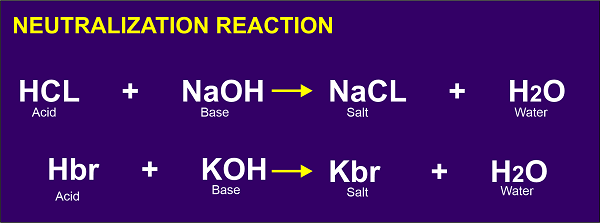
Neutralization Reaction: When a reaction between Arrhenius acid and Arrhenius base occurs, salt and water are formed as products. Such reactions are known as neutralization reactions. Here the bases neutralize the effect of acids.
2. Lewis's theory of bases: Lewis's theory of bases tells about the behavior of the bases in a non-aqueous solution. According to Lewis's theory of bases, a base is a molecule that can donate electrons. Thus, electron-rich speciesare Lewis bases.
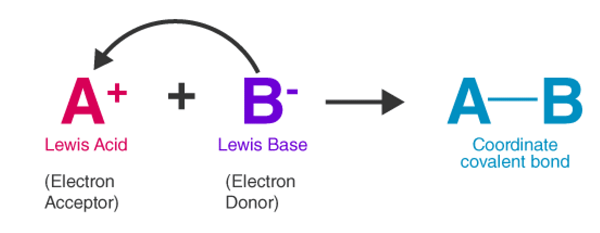
The reaction between Lewis Acid and Lewis BaseWhen a Lewis acid reacts with a Lewis base, a covalent bond formation takes place between the Lewis acid and the Lewis base. As an electron donor, the Lewis base shares the electron with the Lewis acid, thus, forming a covalent bond with the acid.
Here, ammonia, having one lone pair, acts as a Lewis base and shares its pair of electrons with a water molecule, which acts as a Lewis acid (electron pair acceptor); a covalent bond is formed, resulting in the formation of ammonium ion and hydroxide ion.
The hydrogen ion, being electron deficient, acts as a Lewis acid, and the water molecule has two lone pairs, acting as a Lewis base. Water molecule shares their electron with hydrogen ion, thus forming a covalent bond which results in the formation of hydronium ion. 3. Bronsted-Lowry theory of bases: In 1923, a Danish scientist, Johannes Nicolaus Bronsted, and a British scientist, Thomas Martin Lowry, discovered that a Bronsted-Lowry base is a chemical substance that can accept a proton. Thus, a Bronsted-Lowry base is a Hydrogen ion (Proton) acceptor. Thus, this theory was able to explain the behavior of acids or bases in both aqueous as well as non-aqueous solutions. HCl (hydrochloric acid) + H2O (water) → H3O+ (hydronium ion) + Cl- (chloride ion) Here, hydrochloric acid donates a Hydrogen ion (proton) to the water molecule thus, HCl acts as Bronsted-Lowry acid, and H2O acts as Bronsted-Lowry base.
Here, CH3COOH acts as Bronsted-Lowry acid because it donates its hydrogen ion (proton) to water, acting as Bronsted-Lowry base (proton acceptor). 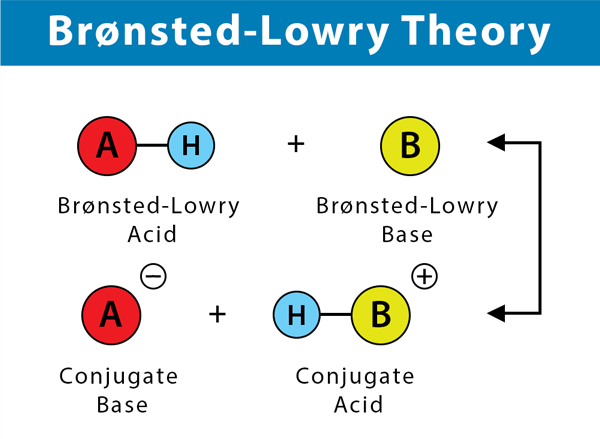
Concept of Conjugate Acids and Bases:Conjugate Base: When a Bronsted-Lowry acid releases a proton (hydrogen ion), the resulting species formed is known as its conjugate base. Conjugate Acid: When a Bronsted-Lowry base accepts a proton (hydrogen ion), the resulting species formed is its conjugate acid. In the below-mentioned reaction, CH3COO- acts as the conjugate base of CH3COOH, and H3O+ acts as the conjugate acid of H2O.
Conjugate Pairs: Acid and its conjugate base or base and its conjugate acid are termed conjugate pairs. In the above reaction, CH3COOH and CH3COO- a conjugate pair as well as H2O and H3O+ is a conjugate pair.
Properties of Bases
NOTE: All alkalis are bases, but all bases are not alkalis.Types of Bases1. Based on the Strength of the Bases
i) Strong Base:A strong base is defined as a chemical substance that can remove a proton from a molecule of a weak acid when an acid-base reaction occurs. A strong base dissociates completely in an aqueous solution, giving one or more than one hydroxide ions per molecule. Some examples of strong bases are:
Lithium Hydroxide (LiOH), Sodium Hydroxide (NaOH), Potassium Hydroxide (KOH), Magnesium Hydroxide Mg(OH)?, etc are examples of strong bases. ii) Weak Base: A weak base is a chemical substance that dissociates partially into its constituent ions in an aqueous solution. It does not completely ionize in water, thus resulting in few hydroxide ions, basic radicals, and many undissociated molecules.
Some examples of weak bases are: Ammonia (NH3), Zinc Hydroxide (Zn(OH)2), Pyridine (C5H5N), Aluminum hydroxide( Al(OH)3), etc. NOTE: Water (H2O) is a weak base and a weak acid because it dissociates to give hydroxide and hydrogen ions.H2O (acid) + H2O (base) → H3O+ + OH- 2. Based on the Acidity of Bases
Acidity of a base: The acidity of a base is the total number of replaceable hydroxyl groups in a base that it can release when dissolved in water or an aqueous solution as ions. The number of OH- ions formed determines the strength of the base. For example, NaOH, when dissolved in water, dissociates into Na+ and OH- thus, the acidity of NaOH is 1. NaOH (aq) (sodium hydroxide) → Na+ + OH- i) Monoacidic Base In a monoacidic base, one hydroxyl ion has the ability to combine with only one hydrogen ion. Examples: Sodium hydroxide (NaOH), Ammonium hydroxide (NH4OH), etc. ii) Diacidic Base In a diacidic base, two hydroxyl ions have the ability to combine with two hydrogen ions. Examples: Calcium hydroxide (Ca(OH)2), Magnesium hydroxide (Mg(OH)2), etc. iii) Triacidic Base In a triacidic base, three hydroxyl ions have the ability to combine with three hydrogen ions. Examples: Aluminum hydroxide (Al(OH)3), ferric hydroxide (Fe(OH)3), etc.
Based on Concentration in Aqueous Solutions
i) Concentrated Base:When the base concentration is highin a solution, it is known as a concentrated base. For example, concentrated NaOH, concentrated KOH, etc. ii) Dilute Base: When the base concentration is relatively lessin the solution, it is known as a diluted base. For example, dilute KOH, dilute NaOH, etc. Some Characteristic Reaction of Bases1. When an alkali is reacted with active metals, hydrogen gas is releasedalong with the formation of a salt. Metal + Base → Salt + Hydrogen gas↑ For example: 2NaOH (aq) +Zn(s) → Na2ZnO2 (aq) + H2 (g) ↑ Note: Because of this property of a base, it cannot be stored in a metallic container.2. Bases have the property of reacting with non-metallic oxides (acidic oxide) to produce salt and water. This reaction is proof that the non-metallic oxides are acidic. Base + Non-Metallic Oxide → Salt + Water 2NaOH + CO2 (g) → Na2CO3 (salt) + H2O 3. Bases react with acid to form salt and water (neutralization reaction). Acid + Base → Salt + Water HCl + NaOH → NaCl (salt) + H2O (water) What is pH?pH: pH, also known as the Potential of Hydrogen is the ability to measure the concentration of Hydrogen ions in a solution. It can also be defined as the negative logarithm of H+ ion concentration.
The range of pH scale is from 0 to 14 and it is used to measure a solution's pH (Hydrogen ion concentration). When the pH of a solution is less than 7, the solution is considered acidic, whereas when the pH of a solution is greater than 7, it is considered basic. When the pH equals 7, the solution is considered neutral. Solutions having pH equal to 0: Strongly acidic (example: Sulphuric Acid) Solutions having pH equal to 14: Strongly basic (example: Sodium Hydroxide (NaOH)) 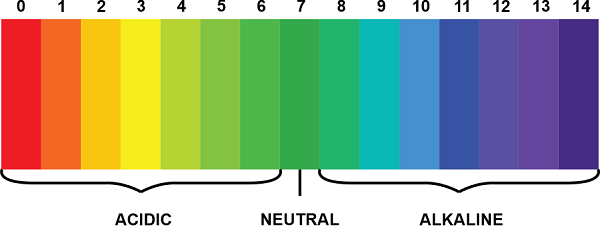
Day-to-Day Uses of Bases
Next TopicWhat is 1 Atomic Mass Unit equal to
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









