Write Things which Dissolve in Water and Which do not Dissolve in Water
In general chemistry, the ability of a substance to dissolve in water is known as solubility. In contrast to this statement, the inability of the substance to dissolve in water is known as insolubility. Solubility and insolubility of substances are the two essential properties of solutes in relation to solvents. Solubility and dissolution are often used as synonymous.
Types of Solubility:
When discussing soluble products or products that dissolve in water, we can define solubility in three types. Since water is considered a universal solvent, things soluble in water need not necessarily be solid, they can be liquid and gases. Therefore, the following are three types of solubility:
- Solubility of solid in liquid
- Solubility of liquid in a liquid
- Solubility of gas in liquid
Factors Affecting Solubility:
Various factors determine the solubility and its extent. The following are the factors that play an essential role in determining the solubility of a solute in the solvent (Water).
- Temperature: Temperature directly affects solubility; it means the higher is the temperature greater will be the solubility. The average observed temperature at which water dissolves solute entirely is 20 degrees Celsius to 100 degrees Celsius, depending on the type of solute. But in the case of gases, the effect is the opposite as the temperature of dissolving gas is increased, and the solubility is decreased.
- Pressure: Similar to temperature, pressure has a similar effect on solubility. As the pressure is increased, its solubility increases. Gases are most affected by pressure, whereas solids have the most minor effect on pressure. Their solubility hardly changes.
- Intermolecular Force of Attraction: Stronger the intermolecular force of attraction in solute more difficult it will be for the solute to dissolve in water. The intermolecular force of attraction is greater among solid solutes followed by liquid solutes, and the least is in gaseous solutes.
Application of Water Solubility:
Water Solubility has many applications in daily life, such as purifying water, making drinks, etc. Every ocean on Earth consists of water mixed with dirt, salt, and other substances. Since this water isn't healthy to drink, it is purified by chemicals that remove the harmful products of the water. Almost everything we drink is a solution because it has chemicals added to it that make it taste better or make it safe for drinking. For example, stirring sugar in a cup of coffee is making a solution. The stirring and heating dissolve sugar faster in coffee and makes it taste better. Another vital application of water solubility is the prevention of food poisoning. For example, chemicals are added to an egg to prevent it from salmonella and to increase its shell life.
Some other famous examples of water solubility are-
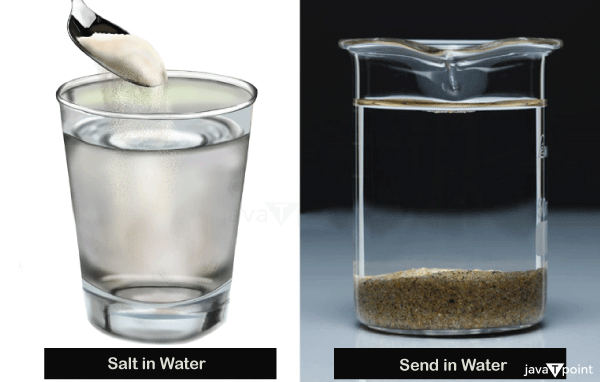
- Salt or sodium chloride is usually soluble in water at 20° C.
- Sugar usually dissolves in water molecules at 20°C.
- Gelatine is soluble in water in the presence of heat.
- Powdered juices are a mixture of sugar, flavourings, and preservatives, usually soluble in water at 20°C.
- Nitrates are commonly found in fertilizers used in agriculture.
- Alcohol like ethyl and isopropyl dissolves in water.
- Wine is a mixture of alcohol and fermented fruits.
- Soap contains carbon, hydrogen, and salt, so it dissolves water.
- Ammonia exists in a wide range of household Chemicals.
- Oxygen dissolved in water is essential for the aquatic world's inhabitants and other living things.
Examples of things that dissolve in water:
- Solids: Salt will dissolve entirely in water. Some parts of the coffee will dissolve, giving brown colour to the water. Sugar will dissolve entirely in water. Food colour will dissolve in water, and flour, medicine, and glucose will dissolve entirely in water.

- Liquids: Alcohol, vinegar, milk, acetone, lemon juice, etc., are some liquids that can dissolve in water.
- Gases: Gases like carbon dioxide and oxygen are most soluble in water.
Examples of things that cannot dissolve in water:
- Solids: Sand, stones, clay, paper, plastic, wood, rubber, glass, metals, cotton, hair, etc.
- Liquids: All non-polar solvents, like oil, petrol, diesel, etc., do not dissolve in liquid but instead form a layer over liquid. Such liquid can be easily separated from the liquid using the proper separation method.
- Gases: Gases like methane, hydrogen, nitrogen, helium, etc., are insoluble in water.

Types of Solution:
Depending upon the amount of solute that dissolves in water, the solution can be divided into two types-
- Saturated Solution: Solutions in which no more solute can be dissolved are saturated solutions. The solvents have reached their maximum capacity to dissolve anything in such solutions.
- Unsaturated Solution: A solution that can dissolve more solute in it, but since the required solution is prepared, no more solute addition is required; hence, in such types of solution, when the saturation point is reached no more solute can dissolve in the solution hence the solution becomes unsaturated.
Conclusion:
The solubility of a solute in water is one of the most important properties of water. Anything that dissolves in the water adds its properties to water which are either visible physically or chemically. The physical changes usually observed are color, density, weight (observed during state change), temperature, etc. Chemical changes include chemical reactions, which often turn the water toxic like the dissolution of chemicals from factories turns water unfit for consumption.
|


 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now










