Uses of ChloroformOverview:Chloroform, or tri-chloromethane (often abbreviated as TCM), is an organic compound with the molecular formula CHCl3 and is known widely as a common solvent. It is a poly-halogen compound which is useful in industry and agriculture. It appears like a colorless liquid, it evaporates quickly into gas and 90% of such emissions are natural in origin. It is also produced by several seaweed and fungi that are believed to provide chloroform in soil, and thus they provide chloroform naturally. In a chloroform molecule, a methane molecule with three hydrogen atoms is replaced with three chlorine atoms, leaving a single hydrogen atom. 
Molecular Representation of Chloroform: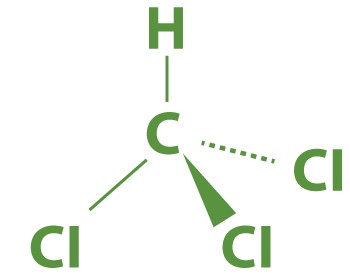
It has properties such as a low boiling point, and it has a low global warming potential. It is non-ignitable however its vapors may burn with green flame. Chloroform is slowly oxidized by air in the presence of light to an extremely poisonous gas, carbonyl chloride, also known as phosgene. It is therefore stored in closed dark colored bottles, which are filled completely so that air is kept out. It is a transparent liquid having a slightly sweet taste and an ether-like odor. It is a naturally occurring compound, but most chloroform that exists in the environment is human-made. It is soluble in water and organic solvents like alcohol and ether. Today, the USA makes almost all the chloroform for making other chemicals. But it also sells or trades some to other countries too. India is one of those countries. Since chloroform does not stick to the soil very well, it can travel down through the soil to the groundwater level. The following properties of the chloroform are being exploited commercially for many applications:
Various Fields of Application of Chloroform:Chloroform is used in many industries. It is released from pulp and paper mills, hazardous waste sites, chlorinated water, and certain landfills. Thus, it enters the environment. It is also found in wastewater from sewage treatment plants and drinking water to which chlorine is added to kill bacteria. Small amounts of chloroform are formed as an unwanted product when chlorine is added to water. Chloroform is used in some refrigerants, solvents, and chemical manufacturing. Worldwide chloroform is also used in pesticide formulations, as a cleansing agent, grain fumigant and in fire extinguishers. Some of the common uses of chloroform have been discussed in detail below: 1. Solvent:Within the laboratory, chloroform is used in both ways, as a solvent and as a chemical agent. It is utilized as an industrial solvent in the extraction and purification of some antibiotics, alkaloids, vitamins and flavors. It was utilized in the past as an extraction compound which can dissolve fats, greases, oils, and different items; as a laundry spot. It is used as a solvent in organic chemistry, photography and the production of dyes, drugs and pesticides. Thus, chloroform acts as a very good solvent. Its hydrogen atom attached to the carbon atom participates in hydrogen bonding, which makes it a good solvent for many materials. It is often used as a solvent for lipids, rubber, alkaloids, waxes, gutta-percha and resins and is also commonly used in NMR (Nuclear Magnetic Resonance) spectroscopy. 2. Refrigerant:Chloroform has good refrigeration properties, it has been used as a precursor to make Freon. This is done by reacting it with a solution of hydrofluoric acid which will fluorinate the CHCl3 molecule and release hydrochloric acid as a byproduct in a chemical reaction known as Swarts reaction. These Freon are usually produced for aerosol propellants, refrigeration and air conditioning purposes. It was found that the majority of the tri-chloromethane produced in the world was used for the production of the Freon which is also called chloro-di-fluoro-methane. 3. Lewis acid:In solvents such as CCl4 and alkanes, chloroform makes hydrogen bonds to a variety of Lewis bases. It is thus classified as a hard acid. 4. Reagent:As a reagent, chloroform is used in many reactions like Phase transfer catalysis, Kharasch addition and Reimer-Teimann reaction. Chloroform reacts with aqueous form of sodium hydroxide to produce di-chloromethane CCl2. Thus, it acts as a useful source of CCl2, which is an intermediate compound formed during the Phase transfer catalysis. Chloroform is used during the Reimer-Tiemann reaction to effect the ortho-formylation of activated compounds (such as phenols), as a result of which aryl aldehydes are produced during reaction. In the Kharasch addition, chloroform forms the (?CHCl2) free radical, which can be used for further reactions in laboratories to obtain the desired products. 5. Anesthetic:Anesthesia is considered a state of controlled and temporary loss of sensation or awareness that is induced for medical or veterinary purposes. The anesthetic qualities of chloroform were first described in 1842. For a number of years from now, chloroform has been used as an anesthetic in surgery by adding 30% ether to it. For example, it is used in dentistry during root canal procedures. How does it work as an anesthetic on Humans?Chloroform effectively makes a human unconscious by disrupting the functions of inter-cellular protein synthesis. Doctors will use a cloth mask with a constant drip of chloroform (or ether) onto it, placed over the nose and mouth with a wire frame. Chloroform was used as an anesthetic during surgery for many years before its harmful effects on the liver and kidneys were recognized. Warning:One has to be extremely careful while using anesthetic property of chloroform. This is because if comparably higher levels of chloroform are used for anesthesia then it may induce anesthesia along with changes in respiratory rate and as a result, some cardiac effects are also likely to occur causing irregular heartbeats. Sometimes, gastrointestinal effects like nausea and vomiting are also observed during such cases. This will ultimately result in effects on the liver and kidneys. Exposure to very high levels can result in death. A fatal oral dose of chloroform may be as low as 10 ml with death due to respiratory or cardiac arrest. Chloroform can still apparently be used only as a local anesthetic and solvent in certain dental endodontic (Gutta-percha root canal) surgery procedures. 6. Food Packaging:It is also useful as an indirect food additive in food packaging materials for adhesive components and it is also used as a raw material for manufacturing food contact materials like containers and wrappers. 7. Manufacturing of Plastic:Chloroform is successfully used in the production of plastics (especially vinyl chloride) and in the manufacture of other plastic-based polymers. 8. FTIR Analysis:FTIR stands for "Fourier transform infrared" and it is the most common form of infrared spectroscopy. The spectrum of pure chloroform is used as the reference or background, and pure cholesterol powder or cholesterol extract from milk products is dissolved in chloroform and used for FTIR analysis in many places. 9. Pesticide Production:Chloroform is used as an intermediate in the manufacture of insecticides and pesticides which are extremely useful in the field of agriculture. 10. Film Production:Chloroform is used to produce polyvinyl acetate films, these PVA films have the most constant mechanical properties as they do not reflect change in properties over the longest time, even if the exposure with temperature is made. Hence, chloroform is recommended for casting films of polyvinyl acetate and thus, it is successfully used as raw material in film production. 11. Fumigant:The chloroform fumigation extraction or CFE is a method used to determine the amount of microbial biomass present in a sample by knowing the amount of carbon and nitrogen present in it, it is determined as Microbial biomass carbon (MBC) or Microbial biomass nitrogen (MBN) by calculating the general increase in extractable carbon (C) and nitrogen (N) respectively. This increase is caused due to microbial destruction during the basic process of chloroform fumigation. For initiating this procedure, the moist soil is exposed to ethanol-free chloroform for 24 hours to kill the indigenous microorganisms. 12. Dry Cleaning Spot Remover:Dry-cleaning means the cleaning of clothes is done without using water and just by using small amounts of organic non-polar solvents. The solvent used for dry cleaning should be such that it can evaporate easily without the involvement of heat to dry the solvent. Chloroform is a volatile compound hence it satisfies this property but can it be used for cleaning? The answer lies here, in chloroform or carbon tetra-chloride, each chlorine atom has two non-bonded electrons which makes net zero dipole charge, thus chloroform is a non-polar compound. The most important thing is to know that according to the law of solubility, like dissolves in like, that is, the polar solvent dissolves polar substances and the non-polar solvent will dissolve non-polar substances. Dirt and grease are also non-polar in nature thus, chloroform being a non-polar solvent will help to remove dirt by forming a bond with sticky dirt substances which allows the dirt to leave the surface of the cloth and thus evaporates along with the chloroform. Hence chloroform is a perfectly suitable solvent for dry cleaning. 13. DNA Extraction:Phenol-chloroform extraction is a named method of extraction used to separate nucleic acids from other cellular substances. In this method, a mixture of phenol, chloroform, and iso-amyl alcohol is added to the given tissue samples. This mixture acts on the sample and easily makes the partitioning of lipids and debris into an organic phase, leaving the DNA in the aqueous phase. Hence, in this manner, the chloroform helps in the separation of nucleic acid. Limitations of using Chloroform:Chloroform can be toxic if inhaled or swallowed. If a person is exposed to chloroform such that he keeps breathing chloroform for a longer period or if he ingests chloroform accidentally, then severe damage to his body organs is likely to occur. It can harm the liver, kidneys, eyes, skin and CNS (Central nervous system) of a person. Skin contact with the liquid form of chloroform can cause a rash or a burning sensation. Dermatitis- a kind of skin disease which is caused in cases of physical contact of skin with chloroform for a longer time. At times the fumes of chloroform or even a single drop of chloroform can cause a burning sensation in the eyes. The carcinogenic nature (cancer-causing nature) of chloroform is still a probability but it is a confirmed teratogenic compound (Compound that causes fetal abnormalities during pregnancy). Now it is clear that the workers in factories dealing with chloroform are at high risk of damage as they are continuously in an environment of exposure to chloroform. The level of damage caused to them depends upon the dose, duration, and work being done by them in these factories. Some examples of workers at risk of being exposed to chloroform include the following:
In these factories and workplaces, exposure to chloroform occurs mainly through breathing or skin contact. As explained earlier, exposure to the vapor of chloroform can irritate the eyes, nose and throat. Even the lower concentrations of chloroform can cause dizziness, fatigue, light-headedness, nausea, confusion and headaches. The International Agency for Research on Cancer (IARC) has found that chloroform may be carcinogenic to humans. Chloroform is thus steadily being replaced by less toxic solvents and may no longer be used in some of these applications. Its use as an inhaled anesthetic during surgery has already been largely discontinued. Conclusion:Chloroform is a colorless compound with a pleasant, nonirritating odor and a slightly sweet taste. Most of the chloroform found in the environment comes from industry. It will only burn when it reaches very high temperatures. Chloroform was one of the first inhaled anesthetics to be used during surgery, but it is not used for anesthesia today, it has been replaced by safer and more versatile materials. It is used as an additive in pharmaceutical preparations. Today, chloroform is primarily used as an organic solvent and as an intermediate in many manufacturing industries. Chloroform lasts for a long time in both the air and in groundwater. Most chloroform in the air eventually breaks down, but this process is slow. The products obtained after the breakdown of chloroform in air include phosgene, which is even more toxic than chloroform, and hydrogen chloride, which is also toxic. Some chloroform may break down in soil. In humans, chloroform affects the central nervous system (brain), liver, and kidneys after a person breathes air or drinks liquids that contain large amounts of chloroform. Breathing chloroform for a short time causes fatigue, dizziness, and headache. If you breathe air, eat food, or drink water containing elevated levels of chloroform, over a long period, the chloroform may damage your liver and kidneys. However, chloroform is a very useful compound but in certain situations, it can be harmful to both humans and the environment, hence its uses are limited and under regulations.
Next Topic#
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









