Give 10 Examples of Amorphous Solids and Crystalline Solids
Solid is one of the states of matter in which molecules are held together by force. The constituent particles of the solid are arranged in two patterns: Regular and Irregular. Solid with a regular arrangement of constituent particles is called a crystalline solid, and with an irregular arrangement of constituent particles is known as an amorphous solid.
Solids are categorized into two types based on the arrangement of their constituent particles: (a) Amorphous solids and (b) crystalline solids.
Amorphous Solids:
Substances having an indefinite and unorganized pattern of lattices are known as amorphous solids. They appear solid but do not have an ordered internal structure hence are called pseudo solid. According to material science, they lack long-range order. Amorphous solids are often synonymous with glassy solids and rubbery solids.
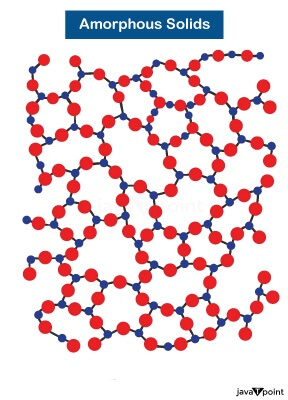
Examples of Amorphous Solids:
- Thin film: Thin film is a layer of highly purified and chemically modified substances with a thickness ranging from fractions of a nanometer to several micrometers. Thin films have become an exciting topic of study after the discovery of amorphous solids.
- Glass/Metallic Glass: Glass is a transparent material. It is a homologous mixture. Glass is not a single compound but a mixture of elements, like sodium oxide, boron oxide, etc. Although some crystalline transparent materials are used to make glass but structurally, glass is an amorphous solid.
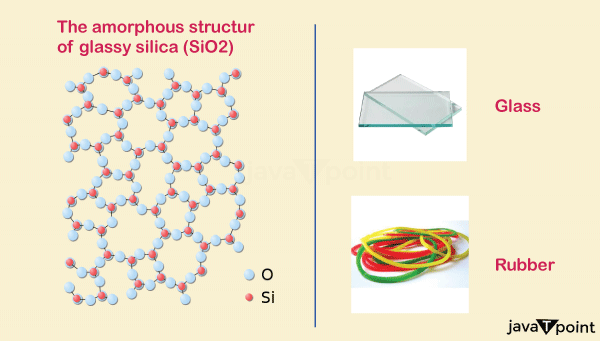
- Rubber: Depending on its source, rubber as an amorphous solid is used as raw material in many industries to manufacture tires, boots, lids, heating utensils, camp fabric, etc.
- Medicines: After glass, medication is the most critical example of amorphous solids. The pharmaceutical industry has shown that amorphous drugs have resulted in more excellent solubility when compared with crystalline solids.
- Wax: For everyday purposes, materials like wax are an example of amorphous solids. They are long alkyl chines in amorphous solids with the chemical formula as- CnH2n+2.
- Gel: Gel is a semi-liquid substance without a three-dimensional arrangement. A gel is a sticky substance and attains a steady irregular solid shape within the gel polymer.
- Photovoltaic Material: Photovoltaic material devices that convert sunlight/solar energy into electricity.
- Plastic: Another example of amorphous solids, Plastics are very lightweight and are formed by clusters of ethene compounds.
- Pitch: A mixture of coal tar and coal stuff is known as pitch. Pitch is a familiar amorphous solid, usually brown in the shade.
- Butter: Butter is an amorphous solid that softens over the high-temperature range.
Crystalline Solids:
Unlike amorphous solids, crystalline solids are solid materials with a symmetrical lattice pattern. Their internal order is well organized. Repeated three-dimensional patterns form the internal structure. Hence, they are called true solids.
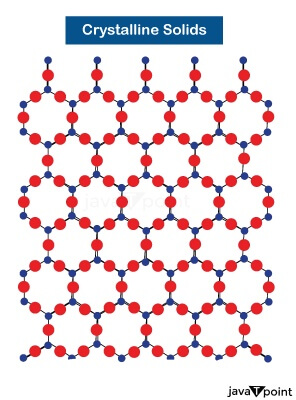
Examples of Crystalline Solids:
- Sugar: Sugar is a pure white crystalline substance widely used for making candies. The crystals of sugar are formed by precipitation.
- Diamond: A diamond is the most famous example of a crystalline solid made of carbon atoms. Diamond exits in its two allotropic forms- Graphite and Fullerene. Crystal-like structure of a diamond is known as a cubic structure.
- Rock: The structure of the rock is known as crystalline due to its presence of crystalline minerals. The most common types of rocks are Igneous, Metamorphic, and Sedimentary.
- Snowflakes: It is the most defined structure of crystalline solids. They have a perfectly symmetrical pattern of internal order made up of water molecules. Snowflakes have a six-sided structure.
- Mica: Mice is a monoclinic mineral containing an octahedral complex aluminum layer. All mica crystalline in the monoclinic system and the principle occurrence of mica are- igneous rocks, metamorphic rocks, and sedimentary rocks.
- Ice cubes: Ice cube is formed from the combination of different types of structure arranged repetitively.
- Calcium fluorides: Calcium Fluoride is a white crystalline solid with the chemical formula CaF The crystals of calcium fluoride are insoluble in water.
- Alum: Alum is also a white crystalline solid with the chemical formula XAl(SO4)2.12H2O.
- Calcite: Calcite is a carbonate mineral with a crystalline structure.
- Table Salt: Table salt, or common salt chemically known as sodium chloride, has tiny crystals of sodium and chlorine bound together through ionic bonds and are obtained by evaporation.
Every solid exists in crystalline or amorphous form, depending on an individual atom or molecule's properties.
|


 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now










