Preparation of Colloidal SolutionsLyophilic colloids are easy to prepare as they have a strong affinity between the dispersed phase and dispersion medium. They can be prepared by shaking, mixing or heating the dispersed phase with the dispersion medium. However, to prepare the lyophobic colloids, special methods are required as the dispersed phase lacks affinity for the dispersion medium in these sols. Let us see how to prepare colloidsThere are two types of methods for the preparation of lyophobic colloids or sols. The first type includes disintegration or dispersion methods and the second type include condensation methods. 1) Disintegration (Dispersion) methodsThey involve physical techniques and are used if the particle size is more than 1000 nm as only the sols in which colloidal particles' size is between 1 nm to 1000 nm can be considered as colloidal sols. So, to bring the particles' size within the colloidal size range we use disintegration methods. The main disintegration methods are described below; i) Bredig's arc method (Electrical Disintegration):This method is mainly used to prepare the colloidal sols of some metals like gold, silver and platinum. For example, purple of Cassius, a colloidal sol of gold is prepared by Bredig's arc method. How does Bredig's arc method work? An apparatus is used in this method. It consists of metal electrodes dipped in the cold water bath. The cold water acts as the dispersion medium. The container that contains the cold water is again placed in a large container that is generally filled with ice. 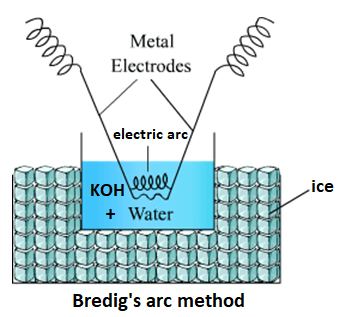
The electrodes are made of that metal whose sol is to be prepared. One electrode, which is connected to the positive terminal is an anode and another one connected to the negative terminal is called the cathode. When the current is passed an electric arc is produced between the two ends of the electrodes. The electric arc is just like an electric spark, the only difference is that an electric spark remains for a short duration, whereas an electric arc stays for a longer duration as long as the current flows in the circuit. The arc produces excessive heat due to which vapours of gold are produced in the cold water. The vapours get condensed due to the cold water and change into colloidal gold particles to make a gold sol. As it is a lyophobic sol that is less stable, so, KOH is added into the sol as an electrolyte. It prevents the gold particles from joining together to form a precipitate. The KOH forms a layer of negative charge around gold particles. So, all particles have the same negative charge so they tend to repel each other, which makes the sol stable and thus precipitation does not occur. ii) Peptisation:In this method, the precipitate is converted into colloidal sol by shaking after adding dispersion medium and electrolyte to it. This method requires three main components that include water, freshly prepared precipitate and an electrolyte to provide stability. In a funnel-like container, water, precipitate and KoH are added. The container is then closed and shaken to prepare the colloid. Let us see how it becomes? The electrolyte contains two types of charges or ions (cations or anions). When we add electrolyte to the precipitate, the precipitate adsorbs one of the charges (positive or negative) on its surface. After adsorbing the same charge the particles of precipitate tend to repel each other and thus the precipitate start breaking into smaller particles of the size of colloidal particles' range. For example, AgCl2 (precipitate) + H2O + AgNO3 (electrolyte) when shaken together in a container form a colloidal sol of Ag. iii) Mechanical dispersion method:In this method, the substance is finely ground and then mixed with the dispersion medium. A stabilizer is also added to it. Then, it is passed through a colloid mill that is made of two heavy metal discs placed one above the other with a small gap between them. These discs rotate in opposite direction at a high speed of around 7000 r.p.m. The shearing effect results in the formation of a colloidal sol and the stabilizers keeps particles from coagulating. The sols of indigo, sulphur, printer ink, toothpaste, ointments, etc., are prepared by this method. 2) Condensation (Aggregation) methodsThese methods involve chemical methods and some other methods which are described below one by one. i) Double decomposition:This method or technique is used to prepare a colloid of arsenic sulphide. The hydrogen gas is passed through a solution of arsenious oxide in distilled water to form the colloid of arsenic chloride as shown by the following chemical reaction. As2O3 + 3H2S → As2S3 + 3H2O In this reaction, double displacement occurs. The arsenious oxide reacts with hydrogen sulphide in which Arsenic goes to Sulphur and hydrogen combines with oxygen to form water. So, arsenic sulphide acts as a dispersed phase and water acts as the dispersion medium and thus a sol is formed. ii) Reduction:This method or technique is used to prepare sols of noble metals like gold, silver and platinum. The reduction of metal takes place in this method as shown in the below chemical reaction 2AuCl3 + 3HCHO + 3H2O → 2Au + 3HCOOH + 6HCl In this reaction, AuCl (chloride of gold) is reduced to gold particles when it reacts with aldehyde and water. Chlorine is separated from Au (gold) and formaldehyde gets oxidised to form carboxylic acid (HCOOH) and water is converted to HCl. In this reaction, reduction of gold occurs by using aldehyde and water and the Gold separated from Chloride becomes the dispersed phase or colloidal particles and HCl becomes the dispersion medium. The reduction of gold can be carried out by using SnCl2 as shown below; 2AuCl + 3SnCl2 → 2Au + 3SnCl4 The above reaction shows that AuCl is reduced to Au and SnCl2 is converted to SnCl4 iii) Hydrolysis:In this method or technique, a sol of ferric hydroxide is prepared by the hydrolysis of ferric chloride by using boiling water as shown in the following chemical reaction. FeCl3 + 3H2O → Fe(OH)3 + 3 HCl Hydrolysis occurs in this reaction. Iron chloride is converted to iron hydroxide as Fe combines with the hydroxyl group in water and Chlorine combines with the hydrogen of water to form HCl. In this reaction, we get the colloidal sol of iron hydroxide Fe (OH)2. iii) Oxidation:The oxidation technique is used to prepare the colloid of sulphur. In this method, a sol of sulphur is prepared by passing the hydrogen sulphide through a solution of sulphur dioxide. For example; SO2 + 2H2S → 3S + 2H2O The above reaction shows the sulphur dioxide reacts with hydrogen sulphide to form water and sulphur. Here, sulphur is oxidised and acts as the dispersed phase and water acts as a dispersion phase. Some other condensation methods, which are not chemical are as follows;i) By exchange of solvent:In this method, a solution of one substance in one solvent is added to another solvent in which that substance is relatively less soluble. The method is used for the substances that are highly soluble in alcohol but less soluble in water. For example, the alcoholic solution of sulphur is added to water to prepare the colloidal sol of sulphur as sulphur is less soluble in water than alcohol. ii) By condensing vapours:In this method, the colloidal sol is prepared by condensing the vapours of a substance. For example, a colloid of Sulphur is formed when vapours of sulphur are added to cold water along with a stabilizing agent like NH4NO3.
Next TopicPurification of Colloids
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










