Classification and Nomenclature of AminesQuick View at Amines:Amines are abundant in nature, they are the major component of proteins, nucleic acids, enzymes and drugs. Amines are a class of organic compounds which contains a basic nitrogen atom and a lone pair. These are actually derivatives of ammonia, developed by replacing one or more of the existing hydrogen atoms of ammonia with a carbon group. They are characterized to have a sp3 Nitrogen atom. The molecular formula of Amine:R3-xNHx Structural Representation: 
Classification of Amines:Classification of amines on the basis of the number of substituents attached to the Nitrogen: Amines are classified as primary (1°), secondary (2°), or tertiary (3°), depending on how many carbon groups are connected to the central nitrogen atom. This classification is as follows: 1. Primary Amines: It is formed by the substitution of one hydrogen atom in ammonia with an alkyl or aryl group, therefore only one carbon group is attached to the nitrogen atom in primary amines. For Example, An example of Primary alkyl amines is methylamine, while an example of primary aromatic amines is aniline. Chemical Structural Formula: RNH2 Molecular Structure of Primary Amines: 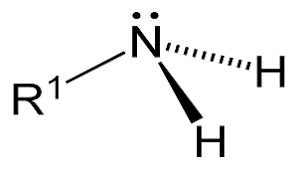
2. Secondary Amines: It is formed by the substitution of two hydrogen atoms in ammonia with an alkyl or aryl group, therefore two carbon groups are attached to the nitrogen atom in secondary amines. For Example, A common example includes dimethylamine. Chemical Structural Formula: R2NH Molecular Structure of Secondary Amines: 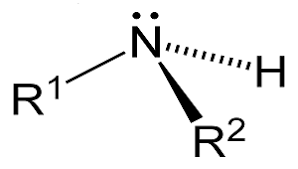
3. Tertiary Amines: It is formed by the substitution of three hydrogen atoms in ammonia with an alkyl or aryl group, therefore three carbon groups are attached to the nitrogen atom in tertiary amines. For Example, Examples of this type of amine are trimethylamine and EDTA. Chemical Structural Formula: R3N Molecular Structure of Tertiary Amines: 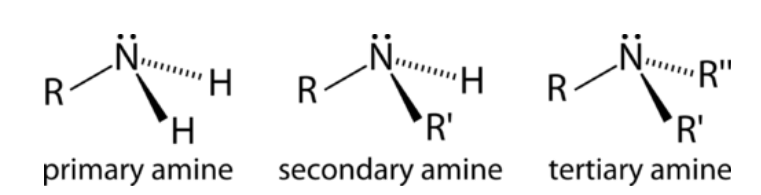
4. Quaternary Amines: In addition to salts of 1°, 2°, and 3° amines, it is possible to have amine cations which contain four alkyl groups attached to a central nitrogen atom, which will always carry a positive charge, regardless of the pH of the surrounding solution. These salts are known as Quaternary ammonium salts. This fourth category of amines, called quaternary ammonium compounds, is prepared by replacement of all four hydrogen atoms of ammonium ion. In this type of amine, the central nitrogen atom has four organic substituents and bears a positive charge. Chemical Structural Formula: R4 N+X- Molecular Structure of Quaternary Amines: 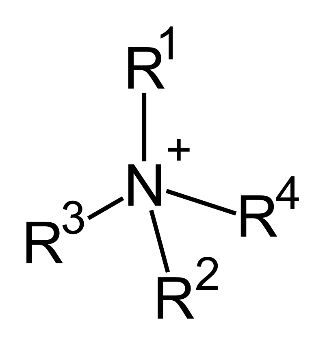
Classification of amines based on the type of substituent attached to the nitrogen: As per the type of substituent, amines are classified as aliphatic amines and aromatic amines.
Classification of Amines based on the Structure of Chain: Amines can also be open-chain or cyclic in structure. Open-chain is one in which the nitrogen is not part of a ring, whereas a cyclic amine is one in which nitrogen is a member of a ring which means the nitrogen is itself aliphatic in nature. For Example, Aniline (cyclic), Ethylamine (open chain). Nomenclature of Amines:Nomenclature of Simple Primary, Secondary and Tertiary amines: The oldest and most typically used alkane series naming system is often termed the trivial naming system. In this system, the common names of amines are obtained by arranging the initial letters of the names of the alkyl substituents on the nitrogen atom in an alphabetical order and then adding the suffix- 'amine' to it. For Example, Methylamine, Ethylamine, Butylamine, etc. Nomenclature of amines using the IUPAC system: An alternative method is also present for the nomenclature of amines, this method substitutes the terminal -'e' of hydrocarbon's name with the suffix-'amine' to represent the functional group ?NH2. Always keep in mind that the largest group attached to the NH2 is chosen as the parent of secondary and tertiary amines, and the rest of the other groups are designated as substitutes. In short, firstly the longest alkane chain in the structure of a given compound is identified, and the "e" ending of the alkane name for the longest chain is replaced with -amine. The substituents attached to the amine group are located by their position numbers. Groups that are attached to the nitrogen atom are located using "N" as their position number. More complex primary amines are named with ?NH2 as the amino substituent. For Example, N-ethyl-N-propylamine, CH3 CH2NHCH2CH2CH3 Nomenclature of Aromatic amines: The cyclic amines are named as derivatives of the parent cyclic compound called aniline. Substituents attached to the nitrogen atom are indicated by using "N-" as their location number. For Example, N, N-dimethylaniline. Nomenclature of Salts of Amines: Salts of amines are named by changing "amine" to "ammonium" and adding the name of the negative ion at the end of the word, (For example Chlorine as the negative ion is written as Chloride at the end of the name of the following example). For Example, Methyl-ammonium chloride Difference between Primary, Secondary and Tertiary Amines:
Identification of Types of Amines:There are a number of tests used to identify types of amine and their structural arrangement, some of them are enlisted below: The identification test for differentiating primary, secondary and tertiary amines is as follows: Hinsberg Test:Hinsberg test is used to identify the primary, secondary, and tertiary amines. Sulfonyl chloride is used as the reagent in the Hinsberg test. During this test, in the reaction, the amines act as nucleophiles and attack the electrophile (sulfonyl chloride). It takes place in an alkaline medium. Observations are as follows:
The identification test for differentiating types of amines as aromatic and aliphatic is as follows: Nitrous Acid Test:The reaction of amines with nitrous acid (HNO2) is another test that classifies the amine not only as primary, secondary, or tertiary but also as aliphatic or aromatic. Certain points to remember before undertaking this test:
Procedure for taking Nitrous acid test in the laboratory:
Specific observations for the identification of amines using the above nitrous acid test are as follows:
Identification test for Secondary Aliphatic Amine:Carbon disulfide reagent test (for secondary aliphatic amines)
Next Topic#
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










