First 20 ElementsThe chemical elements are arranged in a periodic chart based on their atomic number, electron configurations, and chemical characteristics. The first 20 elements of the periodic table are thoroughly described below along with their physical and chemical characteristics, occurrences, applications, and importance. 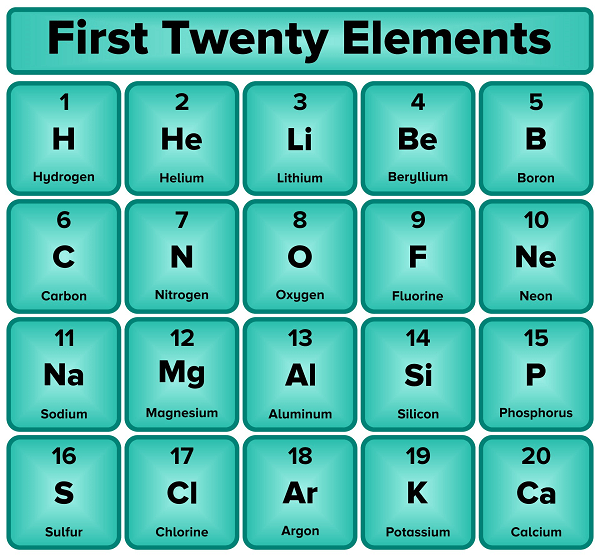
1) Hydrogen (H, atomic number 1)The most prevalent and lightest element in the cosmos is hydrogen. Its centre and orbits each contain one proton and one electron. A colourless, odourless, and extremely flammable gas called hydrogen creates diatomic molecules (H2). It is used as a fuel for missiles and fuel cells, a reducing agent in metallurgy, and the creation of ammonia, methanol, and other chemicals. The fact that hydrogen can exist in three isotopic forms, protium (H-1), deuterium (H-2), and tritium (H-3), each with a different amount of neutrons in the nucleus, makes it unique among the elements. 2) Helium (He, atomic number 2)The centre and orbits of the inert gas helium contain two protons and two electrons, respectively. After hydrogen, it is the second-lightest and most prevalent element in the cosmos. In addition to being used in cryogenics, welding, and as a lifting gas in balloons and airships, helium is collected from natural gas wells. Additionally, it serves as a carrier gas in gas chromatography, a tracer gas in leak detection, and a cooling agent for nuclear plants. Since it has the lowest boiling point (-268.9°C) and cannot solidify under usual circumstances, helium is special among the elements. 3) Lithium (Li, atomic number 3)With three protons and three electrons in its centre and orbits, lithium is a soft, silvery-white metal. It is the least compact solid element and the lightest metal. Lithium is obtained from minerals like spodumene and petalite, which are located in the crust of the earth. Rechargeable batteries, metals, and ceramics all use lithium. Additionally, it is employed in nuclear fusion research, psychiatric medicine, and nuclear reactor coolants. Because it has the greatest specific heat capacity of any solid element, lithium is exceptional among the elements. 4) Beryllium (Be, atomic number 4)The centre and orbits of the hard, grayish-white metal known as beryllium each contain four protons and four electrons. It is one of the most uncommon elements in the crust of the planet and is primarily present in minerals like beryl and chrysoberyl. Workers in industries that use beryllium run the chance of health problems because it is toxic. Aerospace, nuclear reactors, X-ray machines, and other electronic gadgets all use beryllium. Jewelry and other decorative objects also use it. The fact that beryllium has a high melting point (1287°C) and a low mass makes it distinct from other elements. 5) Boron (B, atomic number 5)The centre and orbits of the metalloid boron each contain five protons and five electrons. It is a tough, crystalline solid that is black in colour and can be found in rocks like kernite and borax. Glass, ceramics, and transistor technology all use boron. Additionally, it is a doping substance in semiconductors, a component of detergents, and an insecticide. Neutron detectors also use it. Because of its great hardness and low density, boron stands out among the elements as a special case. 6) Carbon (C, atomic number 6)Six protons and six electrons make up the centre and orbits of the nonmetal carbon. It is the building block of all organic compounds and the fourth most prevalent element in the world. There are many different types of carbon, such as fullerenes, graphite, and diamond. Fossil fuels, plastics, fibres, and other organic things all contain carbon. Additionally, it is used as a propellant, in the manufacture of steel, and to purify water. 7) Nitrogen (N, atomic number 7)A non-metallic substance called nitrogen makes up about 78% of the atmosphere on Earth. At normal pressure and temperature, it is a tasteless, colourless, and non-reactive gas. Numerous organic substances, including DNA, proteins, and other molecules depend on nitrogen. Additionally, it is used to create industrial gases like nitrous oxide, ammonia, and nitrogen oxide in addition to ammonia for pyrotechnics and fertiliser. In the food and beverage business, nitrogen is also used for packaging and preservation. 8) Oxygen (O, atomic number 8)A non-metallic substance called oxygen makes up about 21% of the atmosphere on Earth. It is an extremely reactive, colourless, and odourless gas that is necessary for life. In addition to being used to create combustion engines, steel, and welding, oxygen is also used to create compounds like hydrogen peroxide, ozone, and nitric acid. Additionally, oxygen is used in medical procedures like respiratory therapy and the healing of burns. 9) Fluorine (F, atomic number 9)The non-metallic element fluorine is the most electronegative element in the periodic table, making it extremely reactive and corrosive. It is used to make fluorine-containing substances like hydrofluoric acid, which are then used to make medicines, plastics, and refrigerants. Additionally, toothpaste and fluoride remedies contain fluorine as a component. 10) Neon (Ne, atomic number 10)The atmosphere of the Planet contains traces of neon, an inert gas. It is a gas that is used in gas lasers, lighting, and advertising displays because it is colourless, odourless, and non-reactive. Neon is a refrigerant that is also used in cryogenics, semiconductor manufacturing, and the manufacture of high-voltage apparatus. 11) Sodium (Na, atomic number 11)A soft, extremely reactive metal called sodium is necessary for living. It is used to make chlorine and sodium hydroxide, which are then used to make bleach and other compounds. Additionally, sodium is used to make batteries, as a coolant in nuclear reactors, and as a constituent of metals like NaK and NaLi. 12) Magnesium (Mg, atomic number 12)Life cannot exist without magnesium, a thin, reactive element. Aluminium-magnesium alloys are produced using it and are used in the aerospace and automotive sectors. Magnesium is also used in the manufacture of fertilisers and other chemicals, as well as a component of flares and explosives. 13) Aluminium (Al, atomic number 13)Aluminium is widely used in the building, automotive, and aerospace sectors, aluminium is a popular lightweight and corrosion-resistant metal. It is employed in the manufacture of cans, foils, and other packaging materials as well as alloys like aluminium-magnesium alloys. Additionally, aluminium is a component of many domestic goods and is used in electrical wiring. 14) Silicon (Si, atomic number 14)A metalloid called silicon is frequently used in the creation of photovoltaic cells, semiconductors, and computer chips. Additionally, it is employed in the creation of glass, ceramics, cement, as well as alloys like silicon-bronze and aluminium-silicon alloys. 15) Phosphorus (P, atomic number 15)A non-metallic element called phosphorus is necessary for living. Detergents, fertilisers, and other compounds are made with it. Additionally, phosphorus is used to make steel and to clean effluent. 16) Sulphur (S, atomic number 16)One of the most significant commercial chemicals, sulfuric acid, is created using sulphur, a non-metallic element. Additionally, it is employed in the manufacture of detergents, fertilisers, and other compounds. Additionally, the vulcanization of rubber and the manufacture of batteries both use sulphur. 17) Chlorine (Cl, atomic number 17)The production of PVC and other plastics, as well as the production of bleach and other chemicals, both use chlorine, an extremely reactive non-metallic element. Chlorine is also used to clean wastewater in addition to purifying drinking water. 18) Argon (Ar, atomic number 18)In trace quantities, the atmosphere of the Earth contains the inert gas argon. It is employed in the manufacture of light lamps and metal welding. Additionally, the manufacturing of electronics and the purification of metals both use argon. 19) Potassium (K, atomic number 19)A metal with a significant degree of reactivity, potassium is necessary for life. In addition to being used to make fertilisers, it is also used to make compounds like potassium hydroxide, potassium nitrate, and potassium permanganate. Additionally, potassium is used as a coolant in nuclear reactors and as a component of alloys such as NaK. 20) Calcium (Ca, atomic number 20)A reactive metal necessary for living is calcium. It is employed in the creation of batteries and other electrical devices in addition to cement, glass, and other building components. In addition to being used to make steel, calcium is also used to cure osteoporosis. Importance of Atomic NumberThe amount of protons in the nucleus of an atom of a particular element is known as the atomic number. It is a basic characteristic of an element and establishes its position in the periodic chart. Because it defines an element's chemical properties, the atomic number is significant. An element's atomic number determines its electronic configuration, which in turn determines its chemical behaviour, including its reactivity and capacity to create chemical bonds. In the periodic chart, elements that have comparable atomic weights and electronic configurations also have comparable chemical characteristics, forming families or groups. In order to differentiate between isotopes of the same element that have varying numbers of neutrons in their nuclei, the atomic number is also used. In disciplines like nuclear physics, radiology, and medicine, this difference is crucial. The number of protons in an atom's nucleus defines its spectral and mass signature, and analytical chemistry techniques like spectroscopy and mass spectrometry use atomic numbers to identify elements. What is described by the atomic number in the periodic table?The number of protons in the nucleus of an atom of an element is indicated by its atomic number in the periodic table. The position of each element in the periodic chart is determined by this distinctive identifier for each element. The elements in the periodic table are arranged in columns or groups according to their atomic numbers, with elements with comparable atomic numbers and electronic configurations being grouped together. The number and configuration of electrons in the electron shells or orbitals around the nucleus are determined by the atomic number, which in turn defines the chemical properties of the element. Chemical characteristics such as valence electron configuration, ionisation energy, electronegativity, and reactivity are shared by elements in the same group of the periodic chart. The same element's isotopes, which have the same atomic number but different amounts of neutrons in their nuclei, can be distinguished from one another using their atomic numbers. In general, an element's atomic number is a basic characteristic and a key factor in determining both its chemical properties and where it belongs in the periodic chart.
Next TopicFirst Order Reaction
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










