Some Daily Examples of OsmosisIntroduction:Ever thought why pickles and jams or jellies have a high concentration of salt and sugar respectively? In our everyday life, we see numerous examples of osmosis in nature and surroundings starting from absorption of water by the roots of plants, food preservatives, basic metabolic activities of the cells in the human body, applications in petrology, to aquatic life where the existence of fresh water and marine water fishes within their respectively suitable salt concentrations is maintained through osmosis. Osmosis is a passive process in which a solvent pass from a solution of low solute concentration to a solution of high solute concentration, without any expenditure of energy. The nature of the solvent in such solutions can be gaseous or liquid. It can be referred to as a type of diffusion of water molecules. Osmotic pressure is one of a kind of Colligative properties of an ideal solution. Related Definitions:1. Osmosis: The process of the spontaneous flow of the solvent molecules from the solvent to the solution in such a manner that the flow is from a less concentrated solution to a more concentrated solution through a semipermeable membrane is known as osmosis. 2. Semipermeable Membrane: In Osmosis there is a flow of solvent molecules and that too through a semipermeable membrane, a semipermeable membrane is a type of barrier which allows certain solutes to pass through them and restricts the other, it is, therefore, a kind of membrane that allows the flow of only selective solvent molecules through them. These can be naturally occurring or made-made via chemical processing. Parchment cellophane and the bladder of a goat or pig are good examples of naturally occurring semipermeable membranes. During a number of chemical reactions semipermeable membrane can be prepared, for example, when an aqueous solution of copper sulphate and potassium ferrocyanide is allowed to react in a porous pot, the pores of the pot get filled by the gelatinous precipitate of copper ferrocyanide and serve as a semipermeable or deferentially permeable membrane. Living tissues like a human being's cell walls are the best example of a naturally existing semi-permeable membrane. 3. Solvent: It is a medium (mostly liquid) in which the solute get dissolved to form a solution. 4. Solute: It is the substance that is being dissolved in a solvent to form a solution. It is generally in smaller amounts as compared to the solvent. 5. Solution: It is a liquid combination that is formed as a uniform mixture of solute (small component) and solvent (main component). We can classify solutions into three categories based on concentration, given as follows:
6. Osmotic Pressure: It is the pressure applied to a pure solvent to restrict it from flowing to other solutions by means of osmosis. It is observed that, when a relatively concentrated solution allows the entry of a certain amount of solvent in it, pressure starts developing, ultimately a threshold is reached where the solution will not allow any more amount of solvent to enter in it, this pressure at threshold is called Osmotic Pressure 7. smotic Potential: It is the potential of water molecules to move from a hypotonic solution to a hypertonic solution across a semi-permeable membrane. Types of Osmosis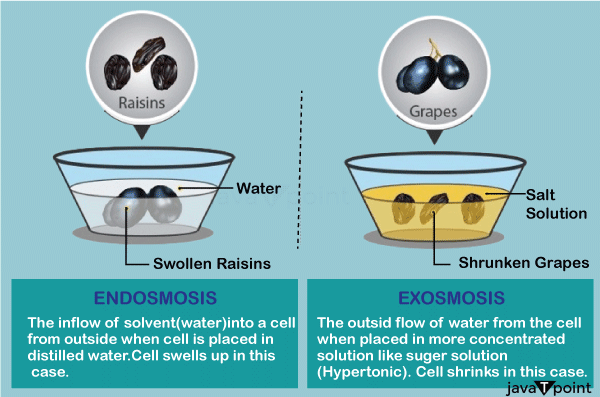
When the solute concentration of the surrounding is less than the solute concentration inside the cell, in such cases, water moves into the cell from the surrounding. Therefore, endosmosis is the type of osmosis which is towards the inside of a cell or vessel. In this case, water (or solvent) will always move into the cell. This type of movement happens because the water potential of the surrounding is higher than that of the cytosol. Usually such kind of flow occurs when a cell is placed in a hypotonic solution. In such cases, the cells may swell. For Example:
When the solute concentration of the surrounding is more than the solute concentration inside the cell, in such cases, water moves from the cell into the surrounding. Therefore, exosmosis is the type of osmosis which is towards the surrounding from the inside of a cell or vessel. In this case, water (or solvent) will always move outside the cell. This type of movement happens because the water potential of the surrounding is lesser than that of the cytosol. Usually such kind of flow occurs when a cell is placed in a hypertonic solution. In such cases, the cells may shrink. For Example:
Daily Examples of Osmosis:a) Preservation of Food: 
The most obvious example of osmosis is food preservation. For this process of preservation to be accomplished, the food substances like pickles, jellies or jam are kept in hypertonic solutions with high salt or sugar solutions respectively. Such hypertonic solution is ideal to keep resistance from microbial growth in food substances and also to prevent any such growths that are favoured by humid environmental conditions. This is possible, as the hypertonic solution favours the movement of water molecules from the cell to the outside region. b) Preservation of Fruit and Meat: Osmosis is very successfully used as a preservative method for Fruits and Meat. However, the preservation strategies for fruits and meat are completely different but the basic process is the same. For the preservation of fruits, the process of osmosis is used to dehydrate the fruit, while for the preservation of meat, the process of osmosis is used to draw salt into it so as to prevent bacterial intrusions into the meat. Sometimes brine is also used to prevent bacterial growth as it is also lethal to bacteria and is hypertonic to bacterial cells. The method of Osmotic dehydration of Fruit is currently trending throughout the world but the Salt Curing method of meat is an ancient method and is no more much in use due to the application and availability of Refrigerators. c) Dialysis: 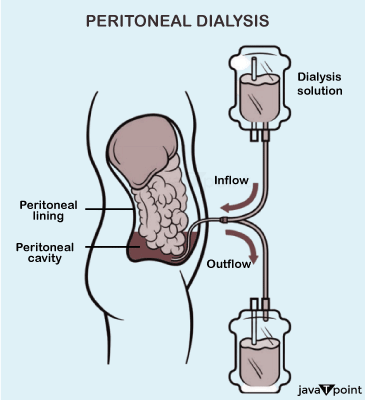
Dialysis is one of the advanced applications of osmosis in the field of medicine and health care. It is used in extreme cases of Kidney failure where an external source of artificial kidneys is required to serve the process of excretion for a patient's body. Dialysis is a process much similar to osmosis, in this process, artificial kidneys are present there to perform the role of excretion of waste products with the help of artificial kidney machines working on the principle of osmosis to remove waste constituents from the body of a patient. In the process of Dialysis, there is a Dialysing membrane which is much similar to the Semi-Permeable membrane of osmosis. The only difference between these two types of membranes is that the dialysing membrane allows the water and certain unimportant elements, mostly salts and small molecules present in the bloodstream to pass through the pores of the membrane. Now these come out and get collected into a tank filled with distilled water. The red blood cells, being too big to pass through this dialysing membrane are allowed to return back to the patient's body. d) Storage of RBCs: The most important cells of the human body, the Red Blood Cells are stored in a plasma solution which is nearly isotonic to these cells. Most hospitals often store the RBCs in a slightly hypertonic solution to prevent them from absorbing water and bursting. e) Intravenous Applications: Physicians use a saline solution which is slightly hypertonic to the patient's red blood cells to inject a drug intravenously into the patient. This is one of the most vital applications of osmosis, important enough to save human life f) Reverse Osmosis: Desalination: Reverse Osmosis is often used for the process of desalination of seawater, i.e. for efficiently converting the sea water into drinking water. This can be done by maintaining an isotonic pressure to the sea level, as a result of which the osmosis is reversed and as per a consequence the pressure in the seawater pushes the water molecules into a reservoir of pure water. 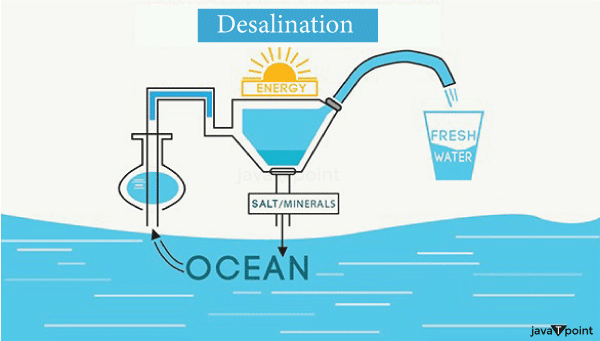
R.O System at Home: In urbanised cities, Reverse Osmosis is often used as R.O, for water purification at home and offices. Reverse Osmosis is a water purification process that removes ions, unwanted molecules and larger particles from drinking water by using a partially permeable membrane. As a result of this method, the solute is kept on one of the membrane's pressurised sides and the pure solvent (pure water) is allowed to pass to the other side, readily fit for drinking. g) Aquatic Life: Sea water and freshwater fishes are able to exist under hyper-saline, saline ad hypo-saline conditions due to the application of osmosis. For instance, Jelly Fishes, a living member of seawater, possess very high concentrations of salt (a solute) as compared to freshwater fishes. This is observed because they have to maintain an isotonic environment with the salty water of the sea. 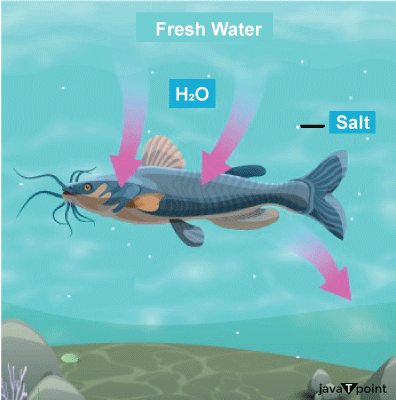
These days synthetic Aquariums are maintaining the lives of fishes in them, away from the natural habitat of fishes, by maintaining the required osmotic pressure into aquariums as per the favourable conditions of fishes. h) Assistance to Plants in Receiving Water: Plants are mostly dependent on the process of osmosis for moving water from their roots to the other parts of the plant specifically leaves. A difference in osmotic pressure is created inside the plant, which is observed as we move towards the leaves from the roots, this is due to the greater solute concentration present at the edges and top of a plant, when compared to their roots. Therefore, a certain amount of Osmotic Potential is developed, which is capable enough to draw the water from the roots in the upward direction in a plant system. 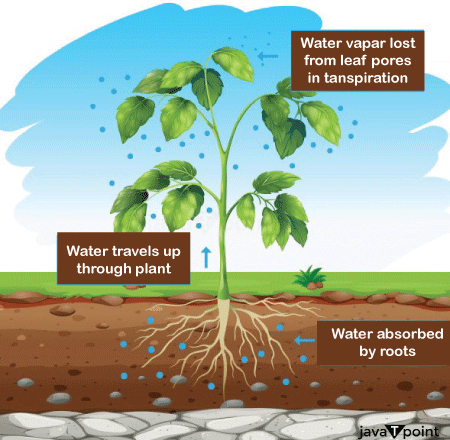
i) Prevents Leaves from Water Loss: Osmosis acts like a boon to plant life by protecting the leaves of a plant, against the loss of water due to evaporation, (also known as transpiration). In a leaf, there are guard cells that are present dispersively all over the surface of a leaf. Each pair of guard cells guards the opening of the stoma, a pore, which, therefore, controls the ability of the pore to open and release moisture to the environment. Whenever there is a deficiency of water the guard cells receive some signals from other cells and this causes them to release their salts (ex. Potassium). A sudden decrease in osmotic potential is thus created as a result of the above events and the water passes out of the guard cells and reaches the other thirsty cells around them. During this time, the guard cells shrink and close the opening called the stomata. This whole process thus results in a decrease in the rate of transpiration through the leaves and prevents the plant from wilting. j) Promotes Plant Growth: In cases of extreme heat and sunlight, the stimulus is sufficient enough to cause the guard cells to draw in certain salts like potassium, thereby increasing the salt concentration, and this ultimately leads to an increase in osmotic potential. The guard cells eventually take over water through osmosis. As a result, the guard cells swell up and opens the stomata, which increases the rate of exchange of gases. Thereby, increasing the rate of photosynthesis and efficient plant growth. Conclusion:Osmosis is a very simple process, which is capable of meeting the very complex demands of all plants, animals and human beings, these demands are very crucial for the accomplishment of their basic needs to survive in everyday life. Starting from a microscopically small cell (like a bacterial cell) to one of the largest living creatures i.e. whale (a fish of seawater) almost everyone is affected by the process of osmosis. It is useful in the existence of aquatic life. Plants are capable to meet their requirement of water via absorption through roots by means of osmosis and this absorbed water is sent to other parts of a plant by generating osmotic potential across the plant body. Preservation of food and Purification of water is one of the biggest industrial applications of osmosis. In the field of Human Health and Medicine, methods like dialysis, intravenous injections and storage of Blood cells, are found to be based on the basic principles of osmosis.
Next TopicWhat is a Chemical Change
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










