Salicylic Acid
Salicylic Acid is naturally found in plants as a growth hormone. It is a monohydroxybenzoic acid; a benzoic acid in which a hydroxyl group is present at the ortho position. It is lipophilic in nature and is obtained from white willow's bark and wintergreen leaves.
It is the conjugate acid of a salicylate and its chemical formula is C7H6O3 or C6H4(OH)COOH. Its IUPAC name is 2-hydroxybenzoic acid. It is also an anti-inflammatory agent and is used as a topical antibacterial agent due to its ability to exfoliate the skin. It is the main ingredient in many topical anti-acne products.
Salicylic acid is an odorless white solid that can be white to light tan in color. The salt and ester derivatives of salicylic acid are called salicylates.
Structure of Salicylic acid
The structural formula of salicylic acid is C6H4(OH)COOH. It can be written as C7H6O3 in condensed form. It has a hydroxyl group (-OH) attached at the ortho position with respect to the carboxylic acid (-COOH) functional group of the benzene ring.
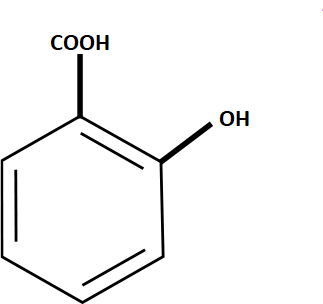
The carbon atoms in the benzene ring of salicylic acid are sp2 hybridized. It dissociates in an aqueous solution to lose a proton from the carboxylic acid which results in the formation of carboxylate ion, -COO-. This ion forms the intramolecular hydrogen bond with the hydrogen atom of the hydroxyl group, i.e., -OH.
Physical Properties of Salicylic Acid
- It exists as white or colorless needle-like crystals at room temperature.
- Its taste is acrid.
- The boiling point of salicylic acid is 211 degrees Celsius.
- The melting point is 315 degrees Celsius.
- It is nearly insoluble in water because of its lipophilic nature.
- It is soluble in organic solvents such as benzene, ethanol and acetone.
- Its density is 1.44 at 20 degrees Celsius and the molecular weight is 138.12 g/mol.
- Its vapor pressure is 8.2 x 10-5
- Its LogP is 2.26
- pH of a saturated salicylic acid solution is 2.4.
- Its pKa (dissociation constant) is 2.97.
- It tends to undergo discoloration when exposed to sunlight.
- It produces irritating fumes and smoke upon degradation.
Chemical Properties of Salicylic acid
-
Formation of Aspirin:
In the pharmaceutical industry, it is used in the production of acetylsalicylic acid (aspirin), which is a widely used analgesic. The salicylic acid reacts with acetic anhydride in an acidic medium which results in the acetylation of the hydroxyl group present in the salicylic acid that produces aspirin.
-
Esterification Reaction:
Salicylic acid when reacts with organic alcohol groups produces new organic chemicals called esters. For example, when salicylic acid reacts with methanol in an acidic medium at a specific temperature, it results in a dehydration reaction in which water is lost and the formation of methyl salicylate (an ester) takes place.
Methods of preparation of Salicylic acid
The two widely as well as commonly used methods for the preparation of salicylic acid are described below:
i) From Phenol:
The steps are given below:
- Phenol reacts with sodium hydroxide to form sodium phenoxide, which undergoes distillation and dehydration.
- The above reaction is followed by a carboxylation reaction using carbon dioxide to form sodium salicylate, which is a salt of salicylic acid.
- The salt formed in the above step is reacted with an acid to form the salicylic acid.
ii) From methyl salicylate:
Methyl salicylate is a common analgesic in the pharmaceutical industry. It is also used to prepare salicylic acid as described below:
The methyl acetate is reacted with sodium hydroxide which forms the sodium salt intermediate of salicylic acid called disodium salicylate. This intermediate is reacted with sulphuric acid to form salicylic acid.
Uses of salicylic acid
There are lots of applications of salicylic acid, some of which are described below:
- It is widely used in the preparation of aspirin, an analgesic. It is also used to prepare another analgesic, which is methyl salicylate and commonly called the oil of wintergreen. Both the products are widely used to alleviate headaches including body aches.
- It also acts as a keratolytic agent, which is used as chemical skin peeling mask to treat skin lesions and extensions from the outer skin surface. It is also used to shed outer skin. However, it should be used as per the instructions of dermatologists.
- Salicylic acid is also used in treating acne and psoriasis. It loosens the keratin content which reduces the pH and softens the skin. It opens the clogged pores and helps in retaining moisture in the skin. It also finds its use in treating wart infections.
- It is used as one of the ingredients in the anti-dandruff shampoos as it prevents the deposition of sebum around hair follicles and skin pores. It also removes dead and flaky skin cells from the scalp thus preventing the chances of dandruff.
- It also has antiseptic properties as it is a popular bacteriostatic agent. Although it does not kill the bacteria but prevents the growth of bacteria, wherever it is applied.
- It is also used in the treatment of ringwork infections, athlete's foot, and a rare genetic skin disorder called ichthyosis that makes skin dry, thick and scaly.
- Salicylic acid is found in many OTC products for skin problems.
Precautions for Using Salicylic Acid
- If you experience an allergic reaction to salicylic acid, you should let your doctor know about it.
- Children' skin absorbs salicylic acid at a higher rate, so it is not advised to use for children under the age of 2.
- Some medicines may not interact well with this acid so tell your doctor about the medicines you are taking before using it.
- Do not apply it on open wounds, cuts, etc.
- Using other skin ointments along with salicylic acid may cause irritation.
- It will cause harm if swallowed, so keep it away from the reach of children.
- Always wash your hands properly after applying it to the affected area.
Salicylic Acid Common Side effects
- Dizziness
- Headache
- Skin irritation
- Difficult breathing
- Skin irritation
- Weakness
Salicylic Acid Gel
Salicylic acid is also available in the form of gel to treat skin problems like pimples, acne, etc. However, your doctor may prescribe it for other reasons. If you are allergic to salicylic acid you should tell your doctor before he prescribes it. Furthermore, store it at room temperature, do not freeze it. Keep it away from heat or open flame and the lid should be tightly closed.
|

 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now










