What is EquilibriumEquilibrium generally refers to a state in which the changes in the system and surroundings are not visible. To achieve this state in chemical reactions, the reactants undergo reversible reactions in which their energy keeps decreasing until it reaches a certain state of low energy where rate of forward reaction is equal to the backward reaction. The reactants tend to stay in this state of low energy unless they are disturbed. This state is known as equilibrium. 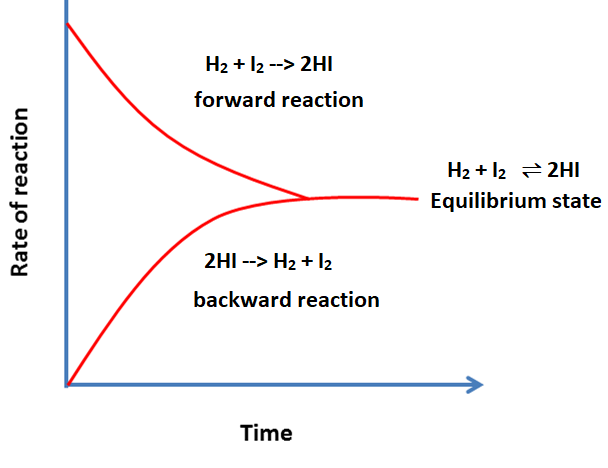
Let us understand it with the following example;There is a liquid in a closed container. Generally, it seems that the liquid level is constant, but it is not true. The liquid molecules with high kinetic energy keep moving from the liquid's surface into the vapor phase and simultaneously a number of molecules from vapor phase with less kinetic energy keep striking the liquid's surface and enter back into a liquid phase. So, the vapor pressure remains constant as there is an equilibrium between the liquid and vapor phase as the number of molecules leaving the liquid phase are equal to the number of molecules returning to the liquid phase. In other words, the rate of evaporation is equal to the rate of condensation. H2O (l) ⇌ H2O (vap) In the above reaction, the two half arrows show the equilibrium or it indicates that both forward and backward reactions are happening at the same time and rate. This type of equilibrium that exists between different phases is known as physical equilibrium. Similar to physical equilibrium, chemical equilibrium also exists. Let us take an example of a reversible chemical reaction wherein reactants A and B undergo a chemical reaction to give C and D as products. A stage comes where the concentration of products increases and the concentration of reactants decreases. It results in the backward reactions along with the forward reaction. At one stage, the rate of the forward reaction becomes equal to the backward reaction which results in the chemical equilibrium as shown below; A + B ⇌ C + D Some examples of reversible chemical reactions that show equilibrium are given below;
As we know in equilibrium, the rate of the forward reaction is equal to the backward reaction. So, if we see the first reaction mentioned above, the rate of formation of NH3 is equal to the decomposition of NH3. So, if we look at the concentration of ammonia, it will remain the same as the rate of its formation is equal to the rate of its decomposition. For example, if the concentration of ammonia is 5gm then after five minutes it will also be 5gm. Similarly, the concentration of the reactants will be the same at this stage. So, the concentration of each reactant and product remains constant at equilibrium. So, this type of equilibrium where reactions are going on but changes in concentration are not visible are called dynamic equilibrium. Characteristics of Chemical Equilibrium
Types of Equilibrium: Based on the rate of reaction
What is Static Equilibrium?It is a type of equilibrium in which the rate of the forward reaction and backward reaction is zero. It is established when there is no exchange between reactants and products and their particles remain in the rest. The transformation of diamond into graphite is an example of static equilibrium. What is Dynamic Equilibrium?In this type of equilibrium, the rate of the forward reaction is the same as the rate of the backward reaction. A dynamic equilibrium is established after the occurrence of a reversible reaction. So, it is seen in reversible chemical reactions wherein the ratio of reactants and products remains the same. This is because the same amount of reactants are converted to products as the amount of the products are converted to reactants. Besides this, the partial pressure of all the reactants and products also remains the same. Examples of Dynamic Equilibrium
Difference between Static and Dynamic Equilibrium
Types of Chemical Equilibrium: Based on the physical state of reactants and productsi) Homogeneous Chemical EquilibriumIn this type of equilibrium, the reactants and products of the chemical reaction are in the same phase. It can be further divided into two types; the first type refers to reactions in which the number of molecules of reactants and products is the same, as shown below; H2 (g) + I2 (g) ⇌ 2HI (g) N2 (g) + O2 (g) ⇌ 2NO (g) The above two reactions show homogeneous equilibrium as all reactants and products are in the same gaseous state. Their molecules are also the same. In the second type of homogeneous equilibrium, the molecules of substances participating in the reaction are not the same as shown below; 2SO2 (g) + O2 (g) ⇌ 2SO3 (g) ii) Heterogeneous Chemical EquilibriumIn this type, the reactants and products are not in the same physical state as shown in the below reactions; CO2 (g) + C (s) ⇌ 2 CO (g) CaCO3 (S) ⇌ CaO (s) + CO2 (g) In the first reaction, carbon dioxide is in the gaseous state, carbon is in the solid state. In the second reaction, calcium carbonate and calcium oxide are solids, whereas, carbon dioxide is a gas.
Next TopicLaw of Mass Action
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










