Lewis Acids and BasesLewis acids and bases are defined based on the transfer of electrons. So, according to Lewis theory of acids and bases and their reactions, a Lewis base is an electron-pair donor, and a Lewis acid is an electron-pair acceptor. So, a Lewis base tends to donate an electron-pair to a Lewis acid to form a product with a coordinate covalent bond. This product is known as Lewis adduct. 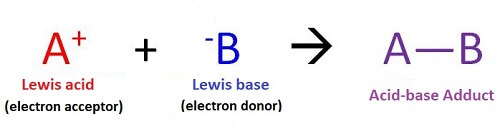
They are named after American chemist Gilbert Newton Lewis. He introduced a broader concept of acids and bases based on electron transfer. It is called the Lewis concept. Lewis AcidThey are chemical species with a tendency to accept an electron pair owing to their empty orbitals. They can be referred to as electrophiles as they are electron-deficient chemical species and thus have a strong tendency to accept electrons. The term 'Lewis Acid' was generally used to denote chemical species with a trigonal planar structure and an empty p-orbital. For example, BR3, where R can be a halide or an organic substituent. Further, there are also some substances that can be considered both Lewis acids as well as Lewis bases as they can accept and donate electron-pair depending on the reaction. For example, water. Some examples of Lewis AcidsThe following chemical species can act as Lewis acid as they can accept electron pairs:
Lewis BaseA chemical species (atomic or molecular) that has the highest occupied molecular orbital can behave as Lewis bases owing to its ability to donate an electron pair to a Lewis acid to form an adduct. Some common Lewis bases include ammonia, alkyl amines, and more. They are typically anionic in nature, and their base strength varies with the pKa of the parent acid. Lewis bases are classified as nucleophiles as they are rich in electrons and tend to donate electron pairs. Examples of Lewis Bases
Further, many molecules with lone pair of electrons are referred to as Lewis bases owing to their ability to donate electron pairs. For example, CH3- and OH-. Chemical reactions between Lewis Acids and Bases
Major Applications of Lewis Acids and Bases
Advantages of Lewis Concept
Limitation of Lewis ConceptIt does not tell about the strength of acids and bases as it does not talk about the ionization process. Lewis acids and bases cannot be arranged in any order based on their relative strengths. The strength of an acid or a base depends on the type of reaction in which it participates. Acids and bases chemical reactions generally occur rapidly, however, there are many Lewis acid and base reactions that are very slow. As a matter of fact, the formation of coordinate covalent bonds (Lewis concept) is a slow process.
Next TopicStrong Acids
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










