What is atomAn atom is the smallest constituent unit or building block of element or matter except for energy. We can say that anything that can be touched physically and occupies space or volume, which is called matter, is made up of atoms such as a chair, table, laptop, plants, bird, dog, etc. So, except for energy, which does not fulfil the definition of matter, everything in the universe is matter and is made of atoms. The word 'atom' is a Greek word whose meaning is 'uncuttable' or 'indivisible'. However, later it was found that atom can be divided into different parts. An atom is made of two regions that include the nucleus, which is located at the centre of the atom and the outer region around the nucleus. The nucleus contains protons and neutrons, whereas, the outer region holds electrons that are located in orbits (energy levels) around the nucleus as shown in the below image. 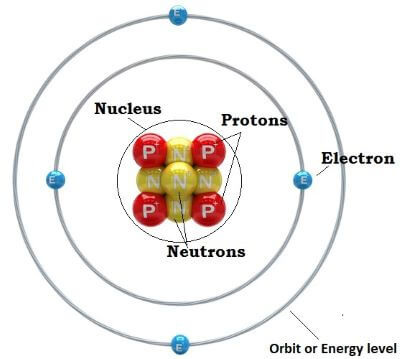
Atom is also the smallest particle of an element, which cannot be broken down further. An atom may or may not exist independently or free. They mostly form ions and molecules that further combine to form compounds. For example, Helium (He) and Neon (Ne) atoms exist independently but hydrogen and oxygen atoms do not exist freely as they are found as H2 ( two atoms of hydrogen are bonded together to make one molecule of hydrogen) and as O2 ( two atoms of oxygen bonded to each other to form one molecule of oxygen) respectively. Molecules are made of one or more types of atoms that bonded together by covalent bonds, e.g. H2O (molecule of water). Atoms cannot be created or destroyed. So, the number of atoms in the reactants before a chemical reaction remains the same in the products formed after the chemical reaction. Scientists have discovered 118 types of atoms that we call elements. History of atoms I How was atom discoveredMaharishi Kanada, a teacher of Sanskrit and a philosopher, was the person who talked for the first time about the atoms in 500 B.C. He said Patharth (matter) is made of tiny identical particles, he called these particles parmanu (atom). Later around 400 B.C. Leucippus and Democritus two Greek philosophers also said about tiny particles of matter and called them atom, which is a Greek word whose meaning is 'indivisible'. Later one more philosopher named Pakudha Katyayama accepted the concept of the atom but he concluded that the atoms may not exist freely or independently and found in a combined state in the form of molecules (a combination of two or more atoms). In 1803, an English schoolteacher, John Dalton gave his theory about atoms based on the data of experiments performed by other scientists. His theory is known as Dalton's atomic theory which states that
A short while later in 1897, JJ Thompson came up with his plum pudding model, he performed a series of experiments and found that atoms couldn't be solid spheres and instead they must be containing negatively charged particles which we now know to be electrons. According to his model, an atom is like a ball of positive charges like a pudding and the electrons are stuck inside it like plums. Thereafter, a few years later in 1909, Ernst Rutherford along with his students made another big discovery. They took positively charged alpha particles and they fired them at a very thin sheet of gold to check that if the positive charge in gold atoms generally spread out as JJ Thompson had proposed with his plum pudding model. As per the plum pudding model, most of the alpha particles should be deflected by the sheet of gold as an atom is like a ball of positive charge as per the plum pudding model. But it did not happen. It was found that most of the alpha particles passed through the gold sheet without any deflection. Whereas, some of the particles were deflected to the side through a small angle and a few were deflected straight back the way they had come. So, based on the findings of Rutherford's experiment, Thompson's theory or plum pudding model was rejected. Based on the results of his experiment, Ernest Rutherford suggested his nuclear model, which proposed that instead of distributed positive charge there was some kind of compact nucleus, which contained all the positive charge of the atom. He also said that the negative charge must exist in the form of a cloud around the central nucleus. He performed an experiment and discovered the negatively charged particles called electrons. He said the electrons revolve around the positively charged nucleus, but he was not able to prove the stability of the atom. Later in 1913, Niels Bohr suggested a solution that the electrons move around the nucleus just like planets revolve around the Sun. He also suggested that the revolving electrons are held in shells that have fixed energy so, electrons do not lose energy while revolving and thus do not strike with nucleus following a spiral path and thus the atom does not collapse. Many experiments supported this model and it is pretty much the same one that we follow now with just a few extra changes. Later, Goldstein discovered the protons that made it clear that the positive charge in the nucleus is actually due to the presence of small particles that we now know as protons and a bit later a guy called James Chadwick discovered neutral particles in the nucleus which we now call neutrons and that is pretty much how we understand the Atom today. How are the atoms named?There is a shorthand representation of the name of an atom or element (made of the same type of atoms), which is known as the atomic symbol. Various scientists tried to give symbols to atoms in the past. However, as of now, the symbols given by ICPAC are followed throughout the world. For example, C for carbon, O for oxygen, N for nitrogen, Ca for calcium, etc. Size of atomAn atom is so small that it cannot be seen with naked eyes or with a high resolution microscope. A human hair is around 500,000 times wider than an atom. However, atoms can be seen using modern technology such as with a scanning tunnelling microscope (STM). The size of an atom is represented or given by its radius, which is called the atomic radius. The atomic radius of an atom is the distance between the nucleus and outermost shell or orbit of an atom. Atom is the smallest particle that takes part in a chemical reaction. It is so small in size that its radius is usually expressed in nanometres (nm). One nm is equal to 10 -9 m e.g. the atomic radius of a hydrogen atom is 10-10m.
Next TopicAtomic Number & Atomic Mass
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









