Hydrochloric AcidHydrochloric acid is a strong acid with the chemical formula HCl. It is an aqueous solution of hydrogen chloride gas. It is the main ingredient of gastric acid, which is produced in the stomach to carry out the digestion of food. It can also be produced for various commercial applications and can be prepared by different processes which also includes dissolving hydrogen chloride gas in water. Chemical Structure of HClIt is an inorganic diatomic chemical made of one hydrogen and one chlorine atom bonded to each other by a single covalent bond. 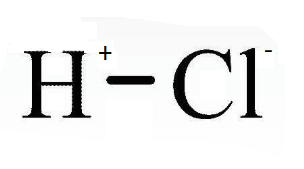
Uses and BenefitsHCl offers a lot of uses, some of its major uses or applications are described below: Steel production: It is widely used in pickling operations that involves removing dust and other impurities from steel, alloys, etc. It prepares the steel for final applications in construction and other projects including its use in car bodies and household appliances. Household cleaners: Being has corrosive properties, it is used as one of the ingredients in household cleaners such as toilet cleaners, bathroom tile cleaners including porcelain cleaners. Pool Sanitation: It is also used for the treatment of swimming pool water in order to maintain an optimal pH. pH Regulation: It is used to regulate the pH of solutions and various other manufacturing and treatment processes in pharmaceuticals, drinking water, beverages, and food items. Produces Inorganic Compounds: Various inorganic compounds can be produced through acid-based reactions using HCl. For example, the formation of iron chloride (III) as shown below: Fe2O3 + 6HCl → 2FeCl3 + 3H2O Iron (III) chloride is used for coagulation and as flocculation agents in drinking water production, treatment of wastewater, and production of paper. Zinc chloride is also produced in this manner, it is used for galvanizing industry and battery production. Calcium chloride and nickel (II) that are used for electroplating are also produced using HCl as shown below: CaCO3 + 2HCl → CaCl2 + CO2 + H2O Production of Organic Compounds: HCl is also required for producing organic compounds such as vinyl chloride and dichloromethane that are used in the production of PVC (polyvinylchloride), ascorbic acid and various pharmaceutical products. Gastric acid: The gastric juice produced in the stomach that helps in digestion also contains HCl. HCl converts inactive pepsinogen into active pepsin that helps digestion by breaking bonds between amino acids, a process called proteolysis. Food Production and Processing: It is widely used in the food industry to process various food products like corn syrups, cookies, cereals, ketchup, etc. Further, it also plays the role of acidifier in saucers, vegetables, juices, etc., to maintain and improve flavor and reduce spoilage. Calcium Chloride: It is used to produce calcium chloride; HCL when reacts with limestone produces calcium chloride, which is used to remove ice from roads. Calcium chloride is also used as a stabilizer in baked food items. Additional Uses: Furthermore, it is also used in the production of fireworks, batteries, gelatin products and in leather processing, and more. Physical Properties of Hydrochloric AcidIt is colorless acid with a highly pungent odor. The other physical properties of hydrochloric acid such as boiling point, melting point, pH, etc., varies based on the concentration of hydrochloric acid in water. Chemical Properties of Hydrochloric Acid
Hazards of Hydrochloric AcidHydrochloric acid is a hazardous liquid, so one must use it with care. It is highly corrosive and in concentrated form, it also produces acidic mists that are also hazardous. If it is inhaled accidentally, it may cause irritation in the eye, nose, and respiratory tract infection. It may also damage the mucous membrane, stomach, esophagus, etc. Further, the Environmental Protection Agency considers HCl a toxic substance. So, protective equipment must be used while using it, some of which are as listed below:
Further, you should have access to an eye-flush station in case someone accidentally comes in contact with it. Storage of Hydrochloric AcidIt should be stored in a cool, dry and well-ventilated area. Further, the container should be tightly closed and it should be away from sources of moisture and incompatible materials like oxidizing agents, organic substances, metals and alkalis. Difference between Hydrochloric Acid and Hydrogen ChlorideTheir chemical formula is the same. They differ from each other in terms of their physical states. Hydrogen chloride exists in a gaseous state, whereas, hydrochloric acid exists in an aqueous solution. Preparation of Hydrochloric AcidIt is prepared by a dissolution process wherein hydrogen chloride gas is dissolved in water. Hydrogen chloride is a molecule that contains a covalent bond and exists as a gas. When it is dissolved in water, it ionizes to form a proton and a chloride ion. The water molecule accepts the hydrogen atom (proton) in HCl and separates it from the chlorine in a process, called dissolution. The hydrogen chloride is produced as a by-product during the large scale production of other chemicals. It is also produced by the combustion of hydrogen in chlorine.
Next TopicMefenamic Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









