Carbolic AcidCarbolic acid, which also goes with the name phenol, is an aromatic chemical molecule. Its chemical or molecular formula is C6H5OH. It belongs to the phenol family of organic compounds and is a white crystalline substance, which is flammable. It is found naturally in the form of a crystalline solid aromatic compound. On a large scale, it is produced through extraction from petroleum. Structure of Carbolic Acid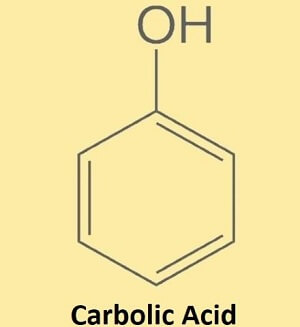
It is an organic compound. Its molecule is made of a phenyl group and a hydroxyl group wherein the phenyl group is bonded to a hydroxyl group (OH) as shown in the below image. It has a six-membered aromatic ring and is bonded to a hydroxyl group directly. Physical properties of carbolic acid:
Chemical properties of carbolic acidThe pi electrons make the ring rich in electrons and thus phenol becomes highly reactive against electrophilic aromatic substitution. Eventually, various groups can be added to the ring through various methods like halogenation, sulfonation, acylation, and more. For example: 1. Addition of Base Phenol when reacts with a base forms the phenoxide anion. It is a deprotonation reaction owing to the removal of the proton or hydrogen ion. The chemical reaction is shown below: C6H5OH + NaOH → C6H5ONa + H2O 2. Addition of Acyl Group Phenol when reacts with ethanoyl chloride at room temperature forms phenyl ethanoate along with hydrogen chloride gas as shown in the below chemical reaction. C6H5OH + C6H5COCl → C6H5OCOC6H5 + HCl 3. Reduction under Zinc Dust Phenol when undergoes distillation with zinc-dust forms benzene and ZnO as a side product. C6H5OH + Zn → C6H6 + ZnO 4. Oxidation of Phenol Phenol when undergoing oxidation forms benzoquinone. It is more prone to oxidation than alcohol. For oxidation, oxidizing agents such as chromic acid, silver oxide, etc., can be used. For example, when chromic acid is used for oxidation, phenol in the presence of sulphuric acid forms benzoquinone. Synthesis of Carbolic AcidSome of the widely used methods for the preparation of carbolic acid are described below: 1. Hock Process In this process, the phenol and acetone are produced from cumene or isopropyl benzene. Oxygen is used for the oxidation of cumene liquid, which forms cumene hydroperoxide which is made to undergo cleavage in acidic media to produce phenol and acetone. 2. Oxidation of Benzene to Toulene The direct production of phenol from the oxidation of benzene is possible theoretically, however, it has not yet been commercialized. C6H6 + O → C6H5OH Further, phenol can be produced from benzene by electrosynthesis, which takes place in an electrochemical cell. 3. Dow's Process In this process, chlorobenzene when heated with aqueous sodium hydroxide at 623K and 300 atm produces sodium phenoxide ion, which in the next step reacts with dilute HCl to form phenol. 4. Hydrolysis of Benzenesulfonate In this process, a strong base reacts with benzenesulfonate. It is the first commercial method for the production of phenol, introduced by Bayer and Monsanto in the early 1900s. The reaction is shown below: C6H5SO3H + 2NaOH → C6H5OH + Na2SO3 + H2O 5. Coal Pyrolysis Phenol can be produced through pyrolysis of coal wherein coal is exposed to high temperatures (400 to 450 degrees Celsius) and that too in the absence of oxygen. The coal goes through chemical and physical separation into different molecules and by-products such as phenol, which can be recovered being a recoverable by-product. Uses of Carbolic AcidThis product should be handled with caution since it can cause chemical burns. Some of the common uses of carbolic acid are as follows:
Health HazardsPoisoning caused by carbolic acid is called carbolism. Its symptoms are as follows:
The general treatment for the carbolism involves washing the stomach with lots of lukewarm water that contains olive oil, castor oil and activated charcoal, with which phenol forms a harmless product. Further, if the carbolic acid happens to fall on clothes, the contaminated clothes should be removed immediately and the skin should be washed and cleaned.
Next TopicKojic Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









